Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Temperature- Or pH-Guided Protein Self-Assembly
Modifications enable alternative mechanisms that yield distinct sizes
by Celia Henry Arnaud
November 9, 2012
| A version of this story appeared in
Volume 90, Issue 46

Dutch researchers have engineered a virus’s protein shell such that it self-assembles via different mechanisms, depending on the conditions. These mechanisms give researchers control over the assembly and disassembly of the resulting structures, which can be used as drug delivery vessels or as nanoreactors.
Jan C. M. van Hest of Radboud University Nijmegen, Jeroen J. L. M. Cornelissen of the University of Twente, and coworkers modified the capsid protein of the cowpea chlorotic mottle virus with a temperature-responsive elastinlike polypeptide (ELP) (J. Am. Chem. Soc., DOI: 10.1021/ja308132z). Unlike the unmodified capsid, the ELP-fused capsid can self-assemble at physiological conditions, at around pH 7.5.
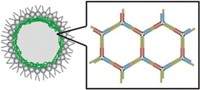
The unmodified capsid’s assembly depends on pH; at around pH 5, it assembles into a hollow shell about 28 nm in diameter.
When the capsid protein is fused to the temperature-responsive ELP, however, its assembly can be governed either by temperature or pH. Below a particular temperature, which can be programmed via the ELP’s amino acid sequence and the salt concentration, the usual pH-controlled assembly mechanism dominates. Above that temperature, an 18-nm capsule assembles regardless of pH.
Both types of capsules have a hollow core surrounded by concentric layers: an outer layer of the capsid protein and an inner one of the ELP. This structure forms because the ELP is fused to the amino terminus of the capsid protein, which is the end that faces inside the particle. “You need to have the ELP inside to stabilize the structure, to give this kind of glue to the capsid proteins,” van Hest says.
“Nature is fond of using such tricks to create ordered materials from a minimum number of building blocks,” says M. G. Finn, a professor at Scripps Research Institute who uses viruses as building blocks for new materials. “In general, this work advances our understanding of how such systems can be engineered; in particular, it allows investigators to create protein nanoparticles with new responsive properties.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter