Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Antibiotic May Sidestep Resistance
Drug Discovery: Bacteria may find agent’s mechanism hard to circumvent
by Stu Borman
May 5, 2014
| A version of this story appeared in
Volume 92, Issue 18

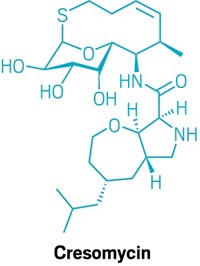
Researchers finally know exactly how the promising antibiotic GE23077 (GE) inhibits an essential bacterial enzyme. They predict that GE’s ability to hit this enzyme in its active site could make it difficult for bacteria to develop resistance to the agent. The study also shows how GE might be conjugated with another antibiotic to make a combination drug the scientists believe might be unusually resistance-proof.
Some bacterial infections are difficult or impossible to treat effectively because bacteria evolve to eliminate antibiotic efficacy, and too few new antibacterial agents have been developed in recent years. The tendency of GE and GE conjugates to evade resistance has yet to be confirmed, but the study points to one possible way to sidestep antibiotic resistance.
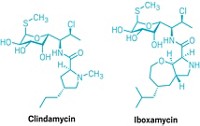
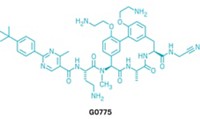
In 2001, an Italian screening study found that the cyclic peptide natural product GE expressed antibiotic activity by inhibiting the essential bacterial enzyme RNA polymerase (RNAP). A 2004 patent covering the molecule is now owned by the biotech company Naicons, in Milan, Italy.
DNA transcription specialist Richard H. Ebright of Rutgers University, Piscataway, and coworkers, including two Naicons researchers, have now figured out that GE does its job by hitting RNAP’s active site (eLife 2014, DOI: 10.7554/eLife.02450). They predict this would make it difficult for bacteria to modify themselves to evade GE while also maintaining RNAP activity.
Rifamycin antibiotics bind to an adjacent site in RNAP and inhibit the enzyme in a different way than GE does. Ebright and coworkers found that a conjugate of GE and a specific rifamycin—rifamycin SV—has higher potency than GE or rifamycin SV alone, and they predict it would evade bacterial resistance more effectively as well. Although GE has trouble getting past cell membranes of some bacteria, conjugating GE to a molecule that more readily enters cells might solve that problem, Ebright says. Rutgers has filed a patent application on GE-rifamycin conjugates.
RNAP expert Robert Landick of the University of Wisconsin, Madison, comments that GE “is probably the first inhibitor that actually binds in the active site, as opposed to around it.” But he isn’t convinced conjugation will solve its cell entry problems. “Making a larger molecule by conjugation, while intriguing, may increase the challenge of cell accessibility,” he says.
Jennifer A. Leeds, executive director of antibacterial discovery at Novartis, notes that “more validation of the hypothesis that GE is unlikely to be eroded by clinical resistance is still needed. But there is potentially huge value in this study because it tells you a lot about the mechanism of RNAP, and the fact that GE and rifamycins have nonoverlapping resistance mechanisms is exciting.”
Ebright says his team now plans to synthesize and evaluate additional GE conjugate inhibitors and develop a total synthesis of GE.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter