Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Turning 1-Alkenes Into 1,4-Diols
Silicon reagent and iridium catalyst selectively transform unactivated C–H bond to alcohol
by Bethany Halford
January 20, 2014
| A version of this story appeared in
Volume 92, Issue 3
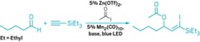
For organic chemists, adding reactive groups to alkenes is a common trick of the trade. Similarly, there are several ways to functionalize the carbon adjacent to an alkene group, which is known as an allylic carbon. But move along the chain just one more carbon—to the so-called homoallylic position—and converting a plain C–H into something more reactive becomes considerably more difficult. Now, chemists at the University of Illinois, Chicago, have come up with a way to activate this otherwise recalcitrant carbon (Nat. Chem. 2014, DOI: 10.1038/nchem.1841). Vladimir Gevorgyan and coworkers found they could convert 1-alkenes to 1,4-diols, thereby adding a reactive handle to the homoallylic position. The researchers first turn the terminal alkene into a hydrosilane. Then, with the help of an iridium catalyst, this hydrosilane reaches around to the homoallylic carbon to form a five-membered ring. Subsequent oxidation produces a 1,4-diol. The researchers demonstrated the 1,4-dioxygenation strategy on several alkene-containing natural products and derivatives.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter