Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
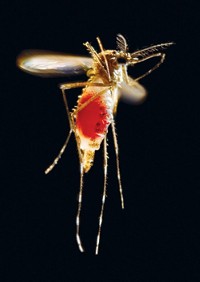
Zika response in full gear
Efforts to combat the outbreak yielded diagnostics and first vaccine trials
by Lisa M. Jarvis
December 5, 2016
| A version of this story appeared in
Volume 94, Issue 48
As travel advisories for pregnant women multiplied, combating the Zika virus was a top priority this year for both government agencies and the drug industry.
“This is the most complicated emergency the CDC has ever handled,” Anne Schuchat, principal deputy director of the Centers for Disease Control & Prevention, told reporters last month at the American Society for Tropical Medicine & Hygiene’s annual meeting. It’s the first time the world has seen a mosquito-borne virus that can be spread sexually or cause birth defects. Moreover, “we have not got a great track record with controlling Aedes aegypti mosquitos,” she said.
The public did get some good news last month when the World Health Organization declared that Zika is no longer a public health emergency. And while health experts stress that efforts to address the virus must continue at full speed, government agencies, health groups, and industry have made some headway in figuring out how to combat it.
Zika by the numbers
28 Babies born in the U.S. with birth defects due to Zika
7 Months Congress took to agree to Zika prevention funding
$1.1 billion Money approved by Congress in September
12 Diagnostic tests granted emergency use authorization by FDA
61 Countries and territories with mosquito-borne transmission of Zika
182 Locally acquired cases of Zika in U.S. states
32,601 Locally acquired cases of Zika in U.S. territories
Sources: CDC, WHO
Prior to the outbreak, there was no FDA-approved test to screen for the virus, which can be difficult to distinguish from dengue, a more common flavivirus. Since February, FDA has issued emergency use authorizations for 12 diagnostic tests.
Ann Powers, chief of CDC’s Alphavirus Laboratory, noted at the tropical medicine meeting that they can be improved. For example, available tests can confirm the virus in about five days; researchers would like to trim that to 36 to 48 hours.
Although a Zika vaccine wasn’t on anyone’s radar prior to the outbreak, three have sped into human trials. Safety studies are ongoing for a purified, inactivated virus vaccine called ZPIV, which was developed by Walter Reed Army Institute of Research, as well as DNA vaccines developed by the National Institute of Allergy & Infectious Diseases and Inovio Pharmaceuticals.
Meanwhile, research into combating the mosquitos themselves continues to lag. “We’ve been very negligent” in finding new ways to control viral vectors, Powers noted. Priorities include the development of new pesticides, studies to determine whether existing pesticides are still effective, and implementing a systemic process for sharing information on the emergence of viral vectors.
“It’s critical not only to Zika, dengue, and chikungunya,” she said, “but whatever might come next—and something will come next.”
MORE ON THIS STORY
- Pharma year in review
- Behind the EpiPen
- Zika response in full gear
- AbbVie's Humira held onto top spot despite biosimilar threat
- After the upset
- Eteplirsen controversy hit FDA
- Pharmaceutical firms saw mixed year in 2016
- Moonshot mania
- Biotech firms managed to go public in rocky year
- Breakup of Pfizer and Allergan shaped the year
- A focus on data



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter