Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Why it’s so hard to make metallic hydrogen
Experimental challenges contributed to the controversy over claims of creating the elusive material
by Mitch Jacoby
June 12, 2017
| A version of this story appeared in
Volume 95, Issue 24

When it comes to having a high-pressure job, diamond anvil cells (DACs) take the cake. By using these niche instruments, researchers can subject solids, liquids, and gases to pressures up to millions of times as high as atmospheric pressure and thereby probe phase transformations, chemical bonding, and other properties of materials under pressures that are inaccessible to nearly every other laboratory technique.
“A diamond anvil cell is basically a fancy nutcracker,” says Isaac F. Silvera, a Harvard University physicist who has been working with high-pressure methods for some 30 years. But instead of crushing nuts between the metal jaws of a glorified pair of pliers, DACs squeeze molecules to extremes between the tips of two diamonds, which is an exceptionally hard material.
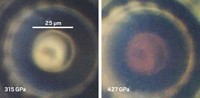
Earlier this year, Silvera and postdoctoral researcher Ranga P. Dias published a study reporting that they had converted gaseous hydrogen to solid, metallic hydrogen by squeezing the element in a DAC to 495 gigapascals, roughly 5 million times as high as Earth’s atmospheric pressure (Science 2017, DOI: 10.1126/science.aal1579).
The study generated considerable excitement because it appeared to end an 80-year quest to form metallic hydrogen. But it also drew a lot of attention because several research groups publicly doubted the findings. C&EN spoke with specialists in high-pressure methods to learn more about why DACs have a reputation for being finicky and how that sensitivity has contributed to the controversy.
The world of high-pressure physics has dreamt of creating metallic hydrogen since 1935, when Physics Nobel Laureate Eugene P. Wigner and Hillard B. Huntington predicted that hydrogen would become a metal if compressed to very high pressure. The prospect of observing that drastic change from insulating gas to solid metal conductor thrills researchers because it represents an extreme of fundamental science.
What’s more, “the potential technological promise of making metallic hydrogen is immense,” asserts Yogendra M. Gupta, a specialist in high-pressure physics at Washington State University. Part of that promise relates to the possibility that metallic hydrogen may turn out to be an exceptional electrical conductor.
Some 30 years after the original 1935 prediction, Cornell University physicist Neil Ashcroft concluded on theoretical grounds that metallic hydrogen would be a room-temperature superconductor. And later studies predicted that the exotic material, if it could be created, might be metastable, meaning that it would remain a superconducting metal after the pressure needed to create it returned to ordinary levels.
If all those predictions hold true—a big if—metallic hydrogen could be a game changer. Unlike copper and other ordinary conductors, superconductors transport electricity without losing energy in the form of heat. To carry out that feat, most superconductors today must be cooled to extremely low temperatures at high cost. Electric motors and other equipment made of room-temperature superconductors, on the other hand, would, in principle, operate at substantial savings energetically and financially. Metallic hydrogen may never end up at the core of electric motors. But understanding how hydrogen transforms into a superconducting metal might pave the way to finding other useful superconductors. In addition to possibly being a room-temperature superconductor, metallic hydrogen may also turn out to be an exceptionally powerful rocket propellant.
For all those reasons, Silvera and other researchers have been trying for years to squeeze hydrogen to extreme pressures in DACs. The diamonds that are central to the devices are cut and polished and look much the same as the ones found in engagement rings, except for their tips. Rather than coming to sharp points, DAC diamonds have flattened tips, which measure a fraction of a millimeter in diameter. These surfaces are known as culets or culet flats and serve as the microscopic jaws in these specimen-crushing vises.

The DAC positions two diamonds such that the surfaces of their culets are almost touching but not quite. The culets meet at a thin gasket made of a hard metal, often rhenium, into which DAC users drill a tiny hole. The hole, which is sandwiched between the culets during an experiment, functions as a specimen holder. As the DAC drives the diamonds toward one another, the culets compress the gasket, which shrinks the hole, subjecting hydrogen or any specimen in the hole to extreme pressure. Because diamond is transparent to many wavelength regions, researchers can use X-rays and visible light, for example, to look through the diamonds and probe the specimen under pressure.
To the uninitiated, the experiment may sound simple. DAC users, however, tell a different story. “It’s very delicate work, and it’s easy to break the diamonds,” says Pamela C. Burnley of the University of Nevada, Las Vegas. She has used DACs for years in geophysics studies that reproduce high-pressure conditions in Earth’s interior.
One of the big challenges is ensuring that the culets remain perfectly parallel as the pressure is ramped up, she says. If they come out of alignment, then as the diamonds are pushed together, the edges that are closest will touch and the diamonds will break.
Steven D. Jacobsen of Northwestern University echoes that view. Jacobsen uses DACs in a range of pressures depending on the nature of the experiment. For example, in a study that produced the first-ever solid-state iron-bismuth compound, FeBi2, Jacobsen worked in the 20- to 30-GPa range, roughly 200,000 to 300,000 times as high as ordinary atmospheric pressure.
He also works at much higher pressures—about 120 GPa—and stresses that those pressures, which he uses to study processes occurring in Earth’s mantle, always spell trouble.
“The diamonds you use to do a 120-GPa experiment don’t come back; they tend to break catastrophically, which ends the experiment,” Jacobsen says.
Scientists aiming for even higher pressures routinely break the costly diamonds before they reach the desired pressure. Yet in their quest to make metallic hydrogen, Silvera and Dias managed to get up to roughly 500 GPa and stayed there for a few weeks before one of the diamonds shattered. How did they do it?
Silvera attributes the team’s success to its specialized diamonds. First, rather than using natural diamonds, as some researchers still do, the Harvard physicist uses synthetic ones grown via chemical vapor deposition (CVD). Second, he coats those gems with a thin layer of alumina.
Silvera explains that the CVD diamonds he uses tend to be free of impurities and lattice imperfections known as inclusions. These types of flaws weaken diamonds, causing them to break before reaching the desired pressure. And the thin layer of alumina functions as a diffusion barrier that prevents hydrogen from penetrating into the diamond, causing it to become brittle and susceptible to breaking.
As Silvera and Dias ramped up the pressure on the hydrogen in their DAC, they monitored the sample’s appearance as it changed from transparent to opaque to highly reflective. Then, by analyzing their data with a mathematical model of reflectivity, they concluded that the shiny appearance of the sample observed at 495 GPa corresponded to hydrogen becoming metallic.
Some researchers voiced objections to the claims, noting, for example, that the team’s method for measuring pressure may have been inaccurate. Rather than using a laser-based Raman spectroscopy technique to monitor pressure throughout the experiment, as is commonly done, the Harvard group used that method only at the highest pressure. Silvera and Dias determined the pressure in the earlier part of the experiment from the number of turns of the “screws” that control the position of the diamonds. Silvera counters that he did so to minimize using powerful lasers that can easily damage the diamonds. And he contends that the mechanical method was properly calibrated.
Other scientists suggested that there is insufficient evidence to attribute the reflections recorded by Silvera and Dias to metallic hydrogen. Perhaps they came instead from the metal gasket or from AlH3, a material that some researchers say may form by reaction of the alumina coating with high-pressure hydrogen. Again, Silvera defends his science, arguing that the reflectivity data point to metallic hydrogen, not the other materials.
Whether the Harvard researchers actually achieved their goal remains an open question. “If they really made metallic hydrogen, that would be wonderful,” says Reinhard Boehler, a physicist at the Carnegie Institution for Science in Washington, D.C. “These experiments are very hard to reproduce,” he adds, but they must be reproduced to address the doubts.
Silvera explains that he published these results before repeating the experiment simply to get the word out quickly, adding that his group is continuing to study hydrogen transformations at extremely high pressures.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter