Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
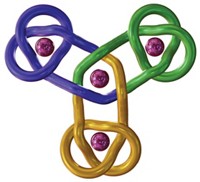
Braiding a molecular knot
Chemists up the complexity of these tangled structures by weaving three strands together instead of just two
by Bethany Halford
January 12, 2017
| A version of this story appeared in
Volume 95, Issue 3

Braiding—the interlacing of three or more strands—seems fairly straightforward, but as any parent of a long-haired child will tell you, it takes skilled hands to master the technique. Braiding a molecule poses an even greater challenge because no hands are nimble enough to entwine structures on such a small scale.
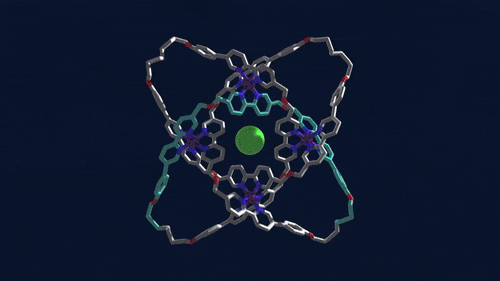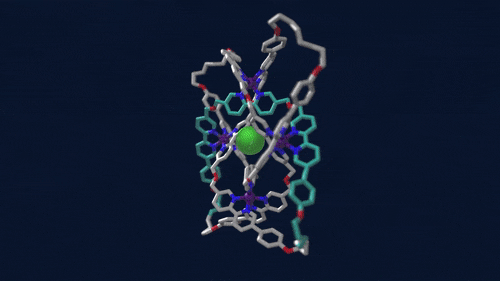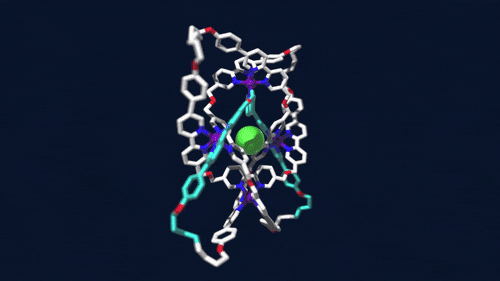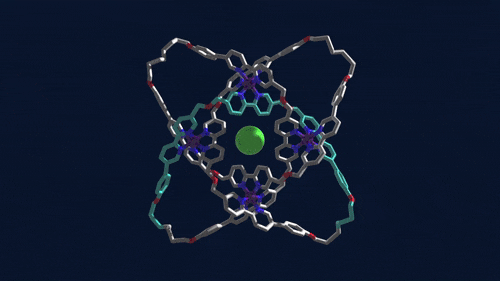
Using iron atoms to guide their molecular building blocks, chemists have now constructed the first braided knot (Science 2017, DOI: 10.1126/science.aal1619). This new knot, crafted by David A. Leigh and coworkers at the University of Manchester, is considerably more complex than previous examples of molecular knots, which were based on just two twisted strands.
Leigh’s group made the 192-atom knot with eight different crossing points by first assembling a circular triple helicate. Helicates are helical complexes that contain metal ions. In this case, four iron ions were used to coordinate three bipyridine moieties on four identical ligand strands.
The chemists heated the metal-ligand mixture, allowing it to arrive at its thermodynamic minimum, which corresponds to the braided structure. They then used metathesis chemistry to link olefins at the ends of the ligand strands together to produce the knot. The tough part, Leigh says, is designing the ligand strand so that it will coordinate the iron ions and so that the strand ends will meet up in just the right way.
“It looks like magic, but it’s not,” says Alberto Credi, an expert in supramolecular chemistry at the University of Bologna, of the synthesis. “This work is an impressive demonstration that sophisticated structural programs can be executed by self-assembly through a careful design.”
“This is the long-awaited step into totally new molecular knots,” adds Amar Flood, an expert in self-assembly at Indiana University Bloomington. “The design with circular helicates is elegant and expedient, and the execution masterful.”
Once the knot structure is assembled, the chemists remove the iron ions, as well as a chloride ion the structure sequesters at its center, to generate a metal-free knot. This molecule, Leigh points out, is chiral. Despite having no chiral centers, it can be made in two distinct forms that are nonsuperimposable mirror images of one another that can be separated using chiral chromatography.
Leigh hopes to use what his group learned from making the braided knot to create even more complex braided knots and possibly even woven polymers. “What we’re really interested in doing is finding general ways that could be used to make whatever sort of knot that you want. There are six billion knots that have been tabulated by mathematicians, and chemists have only been able to make three of them up to now. This is the fourth,” he says. “Clearly there’s a huge unexplored area of chemical space out there.”
This article has been translated into Spanish by Divulgame.org and can be found here.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter