Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biomaterials
Barnacle proteins protect metals from corrosion in salt water
The adhesive proteins stick to steel and form a complex with iron ions in the alloy, creating a protective coating
by Prachi Patel
March 5, 2024

Mussels, barnacles, and other sticky marine invertebrates have been many a researcher’s inspiration for designing novel adhesives. A new study shows for the first time that the proteins in the natural adhesive of barnacles are good at preventing the corrosion of metals in seawater (Commun. Mater. 2024, DOI: 10.1038/s43246-024-00445-z). This finding could aid the development of environmentally friendly anticorrosion paints and coatings.
Metal surfaces of boats and offshore rigs are coated with corrosion-inhibiting compounds to protect them in the high-salt environment of the sea. Commercial inhibitors are organic compounds based on azoles, amines, and phenols. They form tough films on metal surfaces, preventing exposure to seawater, but they can leach toxic chemicals into the environment, harming living organisms.
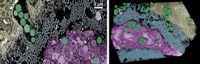
Because barnacles cling to underwater metal surfaces, researchers at Nanyang Technological University investigated whether the crustaceans’ adhesive proteins could form an impenetrable protective layer on metal.
The team genetically engineered bacteria to produce a recombinant protein of the barnacle Megabalanus rosa. Other researchers have made glues for bone or dental repair from this protein because it adheres strongly to inorganic substrates, says materials scientist and engineer Ali Miserez, who led the work.
The researchers immersed steel pieces in a concentrated salt solution that simulated seawater, and they added protein solutions of different concentrations. At concentrations over 5 mg/mL, the protein quickly adsorbed onto the steel surface to form a uniform layer. Spectroscopy analysis and computer simulations suggested that the protein formed a complex with free iron ions in the steel. “This complex effectively covers the metal substrate, preventing corrosion,” Miserez says.
“This is high-risk, high-reward work,” says Nick Aldred, who studies bioinspired coatings at the University of Essex. “It may not work, but if it does, the impact will be large.” A big challenge with biomaterials is that “protein layers tend to become food for bacteria very quickly,” he says, so it will be important to engineer proteins that do not quickly degrade in the environment.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter