Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
The campaign for effective cold and cough medicines
An FDA advisory panel says phenylephrine is a dud. So what cold remedies work?
by Bethany Halford
November 27, 2023
| A version of this story appeared in
Volume 101, Issue 39

Last year, people in the US bought approximately 242 million packages of oral over-the-counter cough, cold, and allergy remedies containing phenylephrine from retail stores, according to sales data compiled by the US Food and Drug Administration. Congested consumers shelled out roughly $1.8 billion on DayQuil, Sudafed PE, and similar products in hopes of relieving their runny noses.

Those millions of pills and elixirs may be pointless, though, at least as nasal decongestants. In September, an FDA advisory panel unanimously agreed that phenylephrine doesn’t work when taken orally. The FDA has the final say on whether it will remove the GRASE designation—short for “generally recognized as safe and effective”—from oral formulations of phenylephrine. The decision could take up to 2 years.
In the meantime, the pharmacy giant CVS is going to stop selling over-the-counter pills and liquids that contain phenylephrine as the only active ingredient. But many over-the-counter remedies contain combinations of drugs, such as phenylephrine plus the fever reducer acetaminophen, and those are likely to remain available for now.
“It’s not a good thing for patients to take medicines that don’t work,” says Eli O. Meltzer, a physician and allergist who has conducted hundreds of clinical trials, including two of oral phenylephrine. Not only is using ineffective medication a waste of money, but it can also delay sick people from seeking treatment, which can lead to a more serious condition. Meltzer says the FDA is responsible for people still spending money on medicines that don’t work.
And he argues that the FDA shouldn’t stop with phenylephrine—cough and cold medicines contain other dud ingredients. “Other now-available, over-the-counter products are not very effective, and they should be restudied,” he says.
Phenylephrine’s popularity
Randy C. Hatton and Leslie Hendeles, experts in pharmaceutical sciences at the University of Florida, have been trying since 2007 to get the FDA to recognize that oral phenylephrine is not effective. And they say other drugs may be similarly ineffective.
Over-the-counter drugs that were once available only by prescription are not the problem, they say. Examples include the allergy medications fexofenadine (Allegra) and cetirizine (Zyrtec), as well as over-the-counter nasal steroids for allergic rhinitis, including fluticasone (Flonase) and mometasone (Nasonex). Those medications have gone through rigorous testing as part of the FDA’s prescription approval process. “The evidence is outstanding for those drugs,” Hatton says.

The problem, they say, is with some—but not all—of the drugs that are part of the FDA’s over-the-counter (OTC) monograph system. That designation refers to drugs that were available over the counter before 1962.
In 1938, Congress passed the Federal Food, Drug, and Cosmetic Act to ensure that a drug is safe. But it wasn’t until the 1962 Kefauver-Harris Drug Amendments that prescription and OTC drugs were required to be both safe and effective. The FDA developed the OTC monograph system to review the efficacy of drugs that were already on the market, but the process has been slow, and in many cases, the data originally used to evaluate those drugs don’t meet modern standards.
When the FDA began reviewing nasal decongestants in 1976, there were three such drugs included in the OTC monograph system: phenylpropanolamine, pseudoephedrine, and phenylephrine. The FDA removed phenylpropanolamine’s GRASE status in 2005 after data showed it was causing hundreds of strokes annually in adults under the age of 50.
Pseudoephedrine came under scrutiny that same year when Congress passed the Combat Methamphetamine Epidemic Act of 2005. Because a simple synthetic step can transform pseudoephedrine into methamphetamine, the law moved medicines with pseudoephedrine to behind-the-counter status. These medicines are still sold without a prescription, but consumers must provide identification to purchase them and are limited in the amounts they can buy.
Drugmakers turned to phenylephrine to fill the public’s demand for OTC nasal decongestants. C&EN reported in 2005 that Boehringer Ingelheim Chemicals was building the first phenylephrine plant in the US, and in 2009 we noted that BASF was adding phenylephrine production to its plant in Minden, Germany.
Phenylephrine has been used medicinally since the 1930s. The compound constricts blood vessels by binding to α-adrenergic receptors. This vasoconstriction prevents mucous membranes’ swelling, which causes congestion.
But when the drug is taken orally, monoamine oxidase enzymes in the gut quickly metabolize phenylephrine to inactive compounds. Recent unpublished data show that less than 1% of a 10 mg dose of oral phenylephrine makes it into the bloodstream unmetabolized. Pseudoephedrine also acts on α-adrenergic receptors, but despite its structural similarity to phenylephrine, 55–75% of pseudoephedrine ends up in urine—unmetabolized.
Phenylephrine’s problems

The University of Florida’s Hendeles says he has known that oral phenylephrine is ineffective since 1993, when he chaired a session on oral decongestants at a clinical pharmacy meeting. “I became aware of one study showing that it wasn’t effective,” he says. That study, which was done in 1971, was never published in a peer-reviewed journal, though. A second study showing that phenylephrine was metabolized to inactive compounds in the gut was published in 1982.
“Even though phenylephrine was on the list of three decongestants back in 1976 that were recommended as safe and effective, drug companies chose not to use phenylephrine,” Hendeles says. He can recall only one product that contained phenylephrine before 2005. All the rest used either phenylpropanolamine or pseudoephedrine. “It was not on my radar screen from 1993 until 2005 because it wasn’t in any products,” he says.
As more oral phenylephrine OTC medications became available, Hatton heard more complaints about their efficacy—or lack thereof—from pharmacists who contacted the Drug Information and Pharmacy Resource Center at the University of Florida. Hatton oversaw that call center, which trained pharmacy students how to respond to questions from health-care professionals.
He learned of Hendeles’s work, and the two began a collaboration to determine if phenylephrine was effective at the available dose. Their analyses determined it wasn’t. So in 2007, they filed a citizen’s petition to the FDA to remove phenylephrine’s GRASE designation.
An FDA advisory panel looked at the available data in 2007 and said that they suggested phenylephrine may be effective. The panel called for more research.
A few years later, Meltzer conducted two clinical trials in collaboration with Schering-Plough and Merck & Co. (which merged with Schering-Plough). One study examined whether increased doses of phenylephrine —up to 40 mg—worked better than the standard 10 mg dose, and the other looked at an extended-release formulation of phenylephrine.
The companies funded the studies because “senior management believed that phenylephrine worked,” says Thomas McGraw, a clinical research scientist who was formerly at Schering-Plough and Merck and who conducted the clinical trials. “They wanted to invest in doing that dose-ranging study and in developing a modified-release phenylephrine,” he says.
But the clinical trials convinced them that phenylephrine was a dud. In both studies, people who took phenylephrine reported the same level of relief from nasal congestion as people who took a placebo. Those studies played a critical role in convincing the 2023 FDA advisory panel that phenylephrine wasn’t effective, McGraw says.
“I admire the companies who were able to publish these data because companies have a vested interest in something called commercial success,” Meltzer says. “And they often don’t want to tell people what doesn’t work.”
Hatton and Hendeles petitioned the FDA again about reconsidering phenylephrine in 2015, citing Meltzer and McGraw’s more recent clinical trial data. The agency was able to begin rereview of phenylephrine as a result of the Coronavirus Aid, Relief, and Economic Security (CARES) Act of 2020, which provided resources, such as more personnel and funding, to help the FDA scrutinize the OTC monograph.
Meltzer praises Hatton and Hendeles for their tenacity. “They looked at the data, and they heard reports from patients. And then, step by step, by their clear persistence, they were able to make this happen.”
Which cold remedies work? And which ones don’t?
Now that the message is getting out that oral phenylephrine isn’t effective, Hendeles says he hopes that pharmacists will be able to recommend medicines that do work. Nasal sprays that contain phenylephrine are still considered effective. Oxymetazoline nasal sprays are also a good option, Hendeles says.
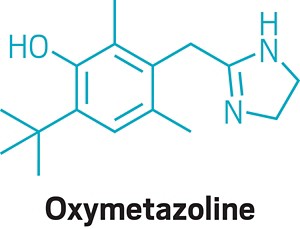
Meltzer, who has spent over 40 years treating nasal congestion, says people should become comfortable taking nasal sprays instead of oral drugs. Nasal sprays, he says, “are generally more effective because you’re putting the medication where the problem is.” He likens the experience of squirting the sprays in the nose to taking a shower: “You get used to the spritz, and it’s not a big deal.”
For people who want to take an oral nasal decongestant, pseudoephedrine is still an option. But Hatton notes that some scientists have questioned pseudoephedrine’s safety, arguing that it can increase blood pressure.
Since more than $1 billion is spent on phenylephrine each year in the US, it seems that drugmakers would be doing research to find an effective replacement. But that’s not the case. “I’m not aware of a single expectorant or nasal decongestant other than the ones that are on the market,” Hatton says.
Malcolm Spicer, who is managing editor of Citeline’s online consumer health resource HBW Insight and has been writing about OTC drugs for 15 years, says that while it may seem that cough and cold remedies would be a prime target for research by drug companies, there isn’t a lot of work in that area.
“Research costs money,” he says, and drug companies need a return on their investment. Until now, companies haven’t had a reason to invest in new ingredients for cough and cold medicines. But depending on what whether the FDA removes phenylephrine’s GRASE designation, companies may have renewed interest in finding new nasal decongestants.

And phenylephrine may be just the beginning for reconsidering the efficacy of common cold and cough medicines. Hatton and Hendeles say there are others that don’t work. They say there’s no good evidence that the prescription drug benzonatate, which is sold under the name Tessalon Perles, works. “I don’t know why it’s still on the market,” Hendeles says.
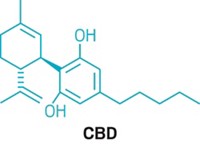
Similarly, they say there’s no convincing evidence that the popular expectorant guaifenesin, which is the active ingredient in Mucinex, is effective. Bruce K. Rubin, an engineer and physician who recently retired from his position as head of pediatrics at the Children’s Hospital of Richmond at VCU, has studied guaifenesin and found it to be no better than a placebo.
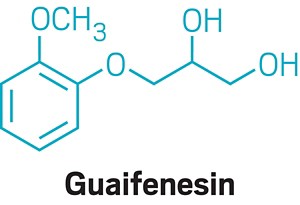

Rubin says the same is true of N-acetyl cysteine, sold as the cough remedy Mucomyst outside the US. In the US,N -acetyl cysteine is available as a dietary supplement, which means it can’t claim to be useful as a medicine and doesn’t have to prove efficacy.
“What makes this devilishly difficult is that the placebo effect is so powerful for cough and cold medications,” Rubin says. Patients frequently report improvement to their symptoms even when they’re taking a sugar pill instead of an active drug.
As a pediatrician, Rubin recommends not giving any cold or cough medications to children as long as the cough is just the result of a cold and not a symptom of a larger problem, such as pneumonia. “Coughing is a very effective way to clear junk out of your airways,” he says. He acknowledges that coughs can be particularly disruptive to sleep, but he says the cough will get better, “and most of the medications won’t make much of a difference.” Rubin tells parents they can give kids over the age of 1 honey to relieve cough symptoms (there’s a risk of botulism with babies less than a year old).
Phenylephrine’s fate
Even with the advisory panel’s recommendation, the final decision about phenylephrine’s GRASE status is up to the FDA, says Adam Cyr, a spokesperson for the agency. “FDA will consider the input of this advisory committee, and the evidence, before taking any action on the status of oral phenylephrine,” he says in an email.
If the FDA determines that oral phenylephrine is not effective, the agency will first issue a proposed order removing phenylephrine from the OTC monograph. “The public would then have the opportunity to comment on the proposed order. If, after considering the comments, FDA continued to conclude phenylephrine is not effective, the agency would issue a final order removing this ingredient from the monograph, and phenylephrine would no longer be considered GRASE,” Cyr says.
If that happens, Cyr says, the FDA “would then work closely with manufacturers to reformulate products as needed to help ensure availability of safe and effective products to treat symptoms of colds or allergies.”
The University of Florida’s Hatton and Hendeles point out that the FDA may still decide not to remove phenylephrine’s GRASE designation. Even so, Hatton says, “it is satisfying to hear that the experts at FDA looked at all the evidence, just like we did, and came to the same conclusion that we have.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter