Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Delivery
Verve pauses gene therapy trial, switches delivery agent
Adverse effects show that LNP choice is tricky, despite the technology’s success in COVID-19 vaccines
by Laura Howes
April 4, 2024
| A version of this story appeared in
Volume 102, Issue 11
Verve Therapeutics is pausing the Phase 1b clinical trial of its cardiovascular disease gene therapy VERVE-101 after a trial participant experienced side effects that the company identifies as being caused by the lipid nanoparticle (LNP) delivery vehicle. The company will now prioritize a therapy in development that uses a different delivery system.

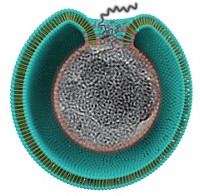
LNPs are the oily bubbles that help deliver the Moderna and Pfizer-BioNTech COVID-19 vaccines, but Verve’s results in gene therapy show that delivery remains a tricky topic to get right, experts say.
Decades of research lie behind the discovery that including ionizable lipids in LNPs can help the globules grab onto cell membranes and even help direct delivery of genetic material to specific tissues. But lipids that perform well in one therapy may not serve in another, and safety can be tied to several factors, says LNP chemist Cecilia Leal of the University of Illinois Urbana-Champaign.
Leal says Verge did the right thing in pausing the trial. But she doesn’t see the announcement as a concern for the LNP field more broadly, saying that lipid-based systems for carrying genetic material are “the most promising delivery agents we have.”
Boston-based Verve launched in 2019 to treat the underlying causes of heart disease with gene editing. In 2021, Verve researchers showed they could lower cholesterol in monkeys by incapacitating the PCSK9 gene with a base editor. That work led to VERVE-101, which includes a guide RNA molecule that recognizes PCSK9 and an RNA molecule that edits the gene, all packaged in an LNP.
Verve said on April 2 that it is now pivoting to VERVE-102. This experimental therapy puts the same RNA components inside an LNP that includes a different ionizable lipid and contains N-acetylgalactosamine (GalNAc) (Nat. Commun. 2023, DOI: 10.1038/s41467-023-37465-1). GalNAc binds to cell surface proteins that are abundant in the liver, where Verve wants to deliver its editor.
Gaurav Sahay, who develops LNPs at Oregon State University, says he hopes the company will also take time to analyze the human data it has from the halted trial to further understand the mechanisms behind the adverse effects, pointing out that there may be multiple variables at play.
Both Sahay and Leal say the setback demonstrates that there is much more to be done to fundamentally understand how LNPs interact in different therapeutic scenarios and how they can be improved. “We all need to keep working,” Leal says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter