Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
Alkaloid biosynthesis inspires tryptophan tag
Attaching a handle to this rare amino acid lets chemists probe its interactions with proteins
by Bethany Halford
March 18, 2024
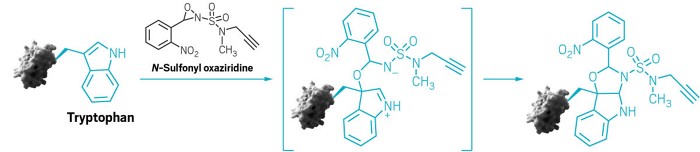
Making chemical handles that latch onto amino acids is a strategy used to make covalent drugs, antibody-drug conjugates, and biochemical probes. Cysteine and lysine are common points of attachment, and methods for modifying methioninehave also been developed. Researchers are now reporting a redox reaction that can tag tryptophan residues.
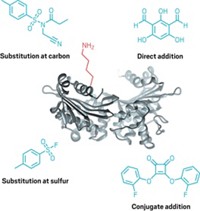
“We’re really interested in developing new types of chemical methods for protein modification and selecting for specific amino acids,” said the University of California, Berkeley’s Christopher J. Chang, who led the work with Berkeley colleague F. Dean Toste. Tryptophan is the rarest of the 20 canonical amino acids, accounting for just 1% of amino acids in proteins. It’s also the only amino acid with a side chain that features indole, which is made of two fused aromatic rings. “It’s like a big mitten,” which is a very different shape from the other amino acids, Chang said.
Inspired by nature’s biosynthesis of indole alkaloids, Chang and Toste’s team developed an N-sulfonyl oxaziridine reagent that reacts selectively and quickly with tryptophan residues that are accessible on proteins (shown). The reaction doesn’t require light, electricity, metal catalysts, or any other reagents. The N-sulfonyl oxaziridine also carries a group that can be further modified to make biological probes or to connect it to other molecules.
Gonçalo Bernardes, a chemical biologist at the University of Cambridge who develops methods for tagging proteins, said it’s exciting to see a strategy for tryptophan-specific modification, as most methods focus on cysteine. “It is likely that this new approach may find other applications, for example to build antibody-drug conjugates modified at tryptophan,” he told C&EN in an email.
Chang and Toste’s team used the tryptophan modification to systematically identify how the amino acid interacts with other amino acids in protein-protein interactions. The researchers mapped tryptophan’s connections to other proteins across a whole proteome via cation-π interactions—its mitten shape essentially shaking hands with other proteins. Strengthening or weakening such interactions could help scientists modify disease-related proteins that are otherwise undruggable.
The researchers also found that the cation-π interactions are important in liquid-liquid protein phase separation—a biological phenomenon that forms organelles without membranes. “We think that this is important for showing not only the proteins that are involved in phase separation but the side chains that are important for making those molecular handshakes,” Chang said.
Xiao Xie, a postdoctoral scholar in Chang’s lab, presented the results at ACS Spring 2024, a meeting of the American Chemical Society, on Sunday in the Division of Medicinal Chemistry. Chang also spoke about the project during a presidential symposium on Monday, and the work was recently published in Nature (2024, DOI: 10.1038/s41586-024-07140-6).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter