Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
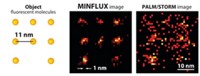
Probe reveals nanometer-scale chemical environment of cell membranes
Adding short alkyl chain to Nile red optimizes it for superresolution microscopy
by Celia Henry Arnaud
August 26, 2019

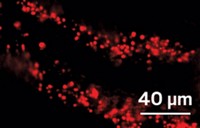
When scientists use superresolution microscopy methods on cells, they usually get just structural information like the sizes and shapes of cellular compartments. By using a new derivative of a conventional dye, researchers can now get specific nanoscale information about the chemical environment of cell plasma membranes. Such information could tell scientists about the order and disorder of the cell membranes, including about highly ordered “lipid rafts.”
Ke Xu, a superresolution microscopy expert at the University of California, Berkeley, collaborated with Andrey S. Klymchenko of the University of Strasbourg to optimize derivatives of the lipid-targeting dye Nile red for superresolution microscopy. They reported the probes earlier this month (Angew. Chem., Int. Ed. 2019, 10.1002/anie.201907690). Xu presented the work this morning in a session in the Division of Analytical Chemistry at the American Chemical Society national meeting in San Diego.
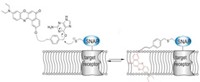
Nile red doesn’t fluoresce in aqueous environments, so in cells, it fluoresces only when it associates with lipids. In addition, it is a so-called solvatochromic dye that is sensitive to the chemical polarity of its surroundings. Thus, the wavelength at which it fluoresces shifts in response to the chemical environment of the lipids like how orderly the greasy molecules are packed together.
In its original form, Nile red associates with lipid membranes throughout cells, which results in strong background signals. The researchers reduced this background and restricted the probe to the cell surface by adding a sulfonate head group with an alkyl chain to the molecule. With a 12-carbon chain, the dye bound quasi-irreversibly to the membrane. That meant it basically got stuck in the on state and eventually became photobleached, which happens when a dye can no longer emit light. This photobleaching makes the long-chain Nile red derivative unsuitable for superresolution methods.

For superresolution microscopy, the researchers needed a probe that would turn on and off. Such behavior is usually achieved with photoswitchable dyes. With the Nile red derivatives, the researchers instead swapped out the 12-carbon chain for a 4-carbon one. That derivative, called NR4A, rapidly associates and dissociates from the plasma membrane, turning itself on and off in the process.
Because Xu could acquire spatially resolved fluorescence spectra concurrently with nanometer-scale images of cells, the researchers could tell how the probe’s fluorescence varied with location and thus whether the lipids at different spots in the membrane were ordered or disordered. “For membrane studies, the chemical polarity is the most important indicator of packing order,” Xu told C&EN. They found that tube-like protrusions on the surface of cells were less ordered than smooth regions of the membrane. The areas around sites where the cell took in cargo from the outside were even less ordered.
The new probe “will allow characterizing lipid membrane heterogeneity with so far unprecedented detail and will open new avenues for cell biological research,” said Christian Eggeling, a superresolution microscopy expert at the Leibniz Institute of Photonic Technology, in Jena.
CLARIFICATION
The story's caption was updated on Aug. 26, 2019, to provide a more expanded explanation of the micrograph and spectra.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter