Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Imaging
Pigment-filled vesicles help track cell-surface signals
Ligands binding to surface receptors can be monitored using the movement of intracellular pigments
by Jyoti Madhusoodanan, special to C&EN
January 30, 2019

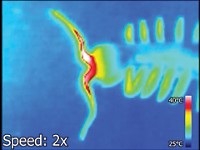
To look deep into tissues, scientists are turning to optoacoustic imaging, a technique in which pigments or nanoparticles introduced into cells absorb light and then emit sound waves, which can be read using ultrasound detectors. A new study shows that the natural pigment melanin can track the activity of a major type of cell-surface receptor with this technique. By implanting melanin-filled cells from frogs into zebrafish, researchers visualized how a molecule bound to its receptor protein in living tissues at a depth greater than fluorescence-based methods could (ACS Sens. 2019, DOI: 10.1021/acssensors.8b01319).
At best, fluorescence-based methods, commonly used to study how proteins work in cells, can retrieve information from approximately a millimeter’s depth, whereas optoacoustic methods can probe several centimeters—the entire span of a rodent brain, for instance. Probes used for optoacoustics usually rely on pigments, gold nanoparticles, carbon nanotubes, or other particles introduced into cells from outside. These probes emit absorbed light energy as sound waves, which can travel greater distances through tissues than light can. For their study, Gil G. Westmeyer of the Technical University of Munich and his colleagues turned to melanin because they knew from previous experiments that it was a good photoacoustic probe. In many species, such as chameleons and cuttlefish, intracellular melanin is encapsulated in vesicles that move within cells in response to hormonal signals acting on a G-protein coupled receptor (GPCR) on the cell’s surface. The GPCR triggers the movement of these vesicles, causing them to aggregate, meaning that vesicle clumping could serve as a proxy for activation of a receptor by its ligand.
Tracking how melanin moves in cells—rather than a frequency shift in the sound waves in a probe’s emitted spectrum—is a new way to monitor a photoacoustic signal, Westmeyer explains.
To demonstrate the method, the team transplanted frog (Xenopus laevis) cells containing melanin vesicles into a particular brain region in juvenile zebrafish (Danio rerio). They treated fish with melatonin, a chemical that triggers vesicle movement via a GPCR, and used raster-scan optoacoustic mesoscopy (RSOM) to measure any change in volume of the regions being imaged. In treated fish, the pigmented area—that is, regions containing the transplanted cells—shrunk approximately 30% in size, suggesting the vesicles had clumped together because of the melatonin signal. Untreated fish showed no change in the size of pigmented areas.
To confirm the method could be used as a reporter for any GPCR, the team engineered cells to carry a synthetic receptor triggered by the drug clozapine. When activated, the receptor set off a signal that instructed vesicles to aggregate. In a small sample of zebrafish transplanted with these modified cells, activating the receptor reduced the pigmented area by 13%, suggesting this cell-based sensor could potentially be engineered to monitor the activity of any cell-surface receptor. The method could be used to study the activity of GPCRs deep inside tissues in model animals to understand signaling pathways or the outcome of activating a particular GPCR.
The work is an exciting proof-of-concept and a novel application of the RSOM technique, says Eric Strohm of the University of Toronto, who was not involved with the study. “Photoacoustic imaging typically has better penetration depth but worse resolution than fluorescence imaging,” he wrote in an e-mail. “But it is still capable of resolving single cells within 3-D space with relatively deep penetration, something that fluorescence cannot do.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter