Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Separations
Microreactor enables rapid testing of green solvents
Strategy reduces volumes and times needed to evaluate CO2-switchable systems
by Lakshmi Supriya, special to C&EN
March 23, 2020

Switchable solvents are a clever idea: They are miscible with a particular substance but then, upon exposure to carbon dioxide, change their chemistry, causing the substance to become immiscible. It’s an easy way to recover both the substance and the solvent without the massive energy input of a distillation, making switchable solvents a boon to green chemistry. A new microreactor containing a droplet of switchable solvent allows these solvents to be characterized in a few minutes (ACS Sustainable Chem. Eng. 2020, DOI: 10.1021/acssuschemeng.9b07304). This requires much less material than established tests and could allow faster identification of suitable solvents for eventual use in solvent recovery processes in the pharmaceutical or chemical industry.
Only a couple dozen switchable hydrophilicity solvents (SHS) have been found, says Philip Jessop of Queen’s University who invented the first switchable solvents and was not associated with this study. “But there are likely thousands waiting to be discovered, some of which may have the magic combination of price, low impact to health and the environment, and performance for applications of industrial interest,” he says. Although switchable solvents are not being used industrially yet, early studies comparing the energy use and environmental impact of switchable hydrophilic solvents compared to standard solvents are very promising, Jessop adds.
SHSs are immiscible in water and become miscible by a simple change in the system. They typically are secondary and tertiary amines. The change in miscibility results from a reversible acid-base reaction upon addition of carbon dioxide.
But evaluating new candidates can take hours. The relatively long switching time “is mainly due to the mass transfer limitations,” because CO2 gas must permeate the contents of a vessel with a limited interface between gas and solvent, says Milad Abolhasani of North Carolina State University and one of the authors of the study. So, Abolhasani and team set out to develop a technique using smaller volumes to speed the mass transfer.
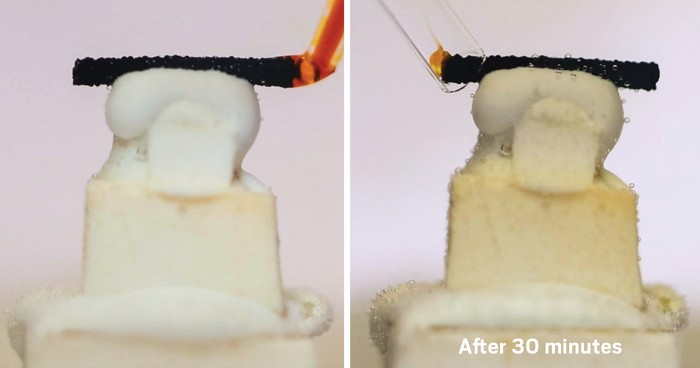
The team’s resulting microreactor has a highly gas permeable tube inside another tube that is impermeable to gas. Using pressurized nitrogen, a water-immiscible droplet of the solvent to be tested blended with toluene is driven into the inner tube along with a droplet of water. CO2 then passes through the annular region between the two tubes and permeates the inner tube, reaching the solvent-toluene droplet and causing the solvent to switch, becoming more hydrophilic and mixing with the water. A camera records the process.
To test the setup, the researchers determined how much of an initially hydrophobic solvent could be extracted in water by making the solvent hydrophilic. Using about 5 µL of several secondary and tertiary amine solvents, they extracted 70-93% of the solvents in water in about six minutes. Achieving a similar efficiency in a traditional batch system used 1000 times more solvent and took 60 times longer. Once carbon dioxide is removed, the solvent can switch back and separates from the water.
The team was able to test how changing different process parameters such as initial solvent concentration, flow velocity, and carbon dioxide pressure affected the rate of extraction of the switchable solvent.
“These microfluidic studies are extremely efficient at generating excellent kinetic and thermodynamic data on the switchable hydrophilicity solvent switching process and separations using the SHS,” says Jessop. They could potentially be used for rapid screening of compounds that may have switchable properties.
CORRECTION
This story was updated on March 25, 2020, to correct the credit for the schematic. The schematic was taken from ACS Sustainable Chemistry & Engineering, not Analytical Chemistry.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter