Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Structural Biology
Laser power changes observed ultrafast protein dynamics
If you want to see natural ultrafast protein dynamics, turn down the laser power, says lead investigator
by Fionna Samuels
February 27, 2024
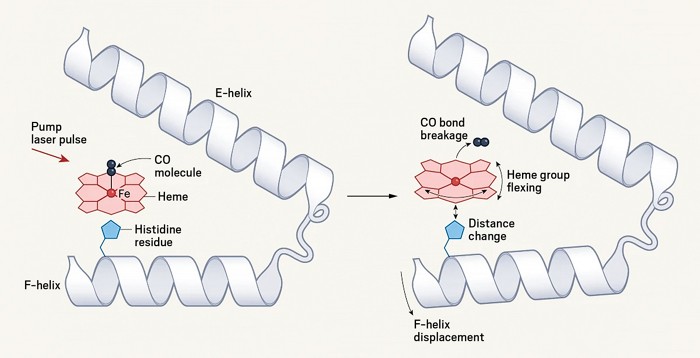
When researchers from the Max Planck Institute for Medical Research performed the first ultrafast x-ray crystallographic experiments on myoglobin in 2015, they didn’t realize they had done the wrong experiment. By cranking up the power of the x-ray free-electron laser to ensure usable diffraction patterns, lead investigator Ilme Schlichting says they “suddenly ended up in the wrong [excitation] regime without noticing.”
Instead of observing a single photon excitation pathway that mirrored myoglobin’s natural dynamics, the laser had hit hard enough to induce multiphoton absorption. This raised the question, were the oscillations they saw in the protein artifacts of this more energetic excitation? Now, Schlichting and her team have redone the experiment at a lower power to check (Nature 2024, DOI: 10.1038/s41586-024-07032-9).
The results surprised Schlichting. “We expected some tiny changes in the dynamics, but what we see is a major change with the carbon monoxide,” she says. Unlike the immediate photolysis they observed at high laser power, the reaction took several hundred femtoseconds longer at low power. The group modeled their observations and attributed their results to two different reaction pathways, the latter likely being more representative of the true reaction, she says.
However, the myoglobin only oscillated slightly differently with low power. This reassures Richard Neutze, a professor of biochemistry at University of Gothenburg. Although unaffiliated with the group, he did review the work prior to publication and wrote a corresponding perspective on the implications of the results. “The work is very important because it’s showing that we weren’t completely wrong before,” he says. Essentially, past high-power experiments were imperfect but still provided valuable big-picture insights into protein dynamics. “On the other hand,” Neutze says, “the authors also show that if you’re really interested in the ultrafast chemistry, then it is important to do the experiment correctly because there are subtle differences in the mechanism, and they matter.”
Ultimately, Schlichting says researchers just need to be transparent about the regime they’re working in. These experiments are inherently challenging. “Sometimes you either go home without any data or you do it in a multiphoton regime,” she says, “but then you should just be honest about it.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter