Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Dynamic Insights into Host-guest Behavior
Mass spectrometry technique reveals how guests dissociate from noncovalent dendritic assemblies
by MICHAEL FREEMANTLE
July 5, 2004
| A version of this story appeared in
Volume 82, Issue 27

Tandem mass spectrometry--a technique that employs two mass spectrometers connected in series--is being used by chemists at Eindhoven University of Technology, in the Netherlands, to investigate supramolecular interactions in the gas phase.
In recent work, the group used the technique to study aggregates consisting of a multivalent dendrimer host for guests based on urea acids or tripeptides and to provide information on their dissociation [Angew. Chem. Int. Ed., 43, 3557 (2004)]. The group consisted of chemistry professor E. W. (Bert) Meijer, graduate student Maarten A. C. Broeren, senior lecturer Marcel H. P. van Genderen, mass spectrometry specialist Joost L. J. van Dongen, and visiting Danish scientists Michael Pittelkow and Jørn B. Christensen.
It's a challenge to observe supramolecular architectures as individual objects, Meijer says. "In particular, the issue of multivalency [binding of multiple guests by a host structure] and how molecules come together in a supramolecular architecture or object is of great biological significance. Almost all biological interactions have multivalent character," he adds.
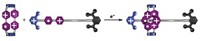
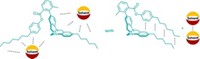
The compounds that Meijer's group studied are based on third-generation adamantylurea-substituted poly(propylene imine) dendrimers. They are good hosts for molecules based on urea acids or tripeptides in solvents such as chloroform. The guests are bound at the periphery of the dendrimer by urea-urea hydrogen bonding or by ion-ion electrostatic interactions.
The group can couple two or three different guests to the same dendrimer molecule and also generate multicomponent libraries of the complexes.
"We observe statistical combinations of different guests bound to the host," Meijer says. "We can see not only different amounts of guests bound to the dendrimer but also different combinations of guests. For example, there are 10 different combinations for the complex containing three guests. As the guests are not covalently bound, the system is dynamic."
The Eindhoven team uses electrospray ionization mass spectrometry (ESI-MS) to transfer the large dendritic aggregates from solution to the gas phase. The solution is first subjected to a powerful electric field that vaporizes the solution and generates ions. The ions are separated from solvent and buffer agents and then analyzed using the mass spectrometer.

The ESI mass spectrometer is separated from a second mass spectrometer by a high-pressure cell in which collision-induced dissociation (CID) of ions with a specific mass takes place. The mass-selected ions are bombarded by neutral gas atoms, for example, argon atoms.
"AS THE IONS collide with the gas molecules, a portion of the ions' kinetic energy is converted to internal energy in the ions," Meijer explains. "The increase in internal energy makes it unstable and drives fragmentation reactions that occur prior to leaving the collision cell. The fragment ions are analyzed in the second mass spectrometer."
The level of fragmentation can be varied by varying the voltage used to accelerate the mass-selected ions before their insertion into the collision cell.
"The amount of internal energy that is taken up by the ion depends on its kinetic energy, so by accelerating the ion before insertion, more severe fragmentation can occur," Meijer observes. "In our case, the technique is used to look at the stability of aggregates in the gas phase rather than the stability of single ions.
"We can use the technique to study molecule-molecule interactions in the absence of a solvent," he continues. "By systematically changing the guests--for example, from sulfonic acid to phosphonic acid or from guests with urea groups to guests without them--we can probe the binding strength of the guests and the influence of secondary interactions such as hydrogen bonding and electrostatic interactions."
CID-MS analysis of the dendritic aggregates reveals that the guest molecules dissociate from the dendrimer one by one in a highly controlled way and that the guest molecules dissociate slightly more easily when fewer of them are bound.

Steven C. Zimmerman, chemistry professor at the University of Illinois, Urbana-Champaign, points out that most classic techniques, such as solution NMR spectrometry, would likely show broad or average peaks and provide little information about such aggregates.
"OVER THE PAST several years, supramolecular chemists have found mass spectrometry to be a useful tool for inferring what is happening in solution," he comments. "Meijer and coworkers have taken this to a new level by analyzing a dynamic library of noncovalent assemblies with a combination of ESI and CID. They not only see all of the assemblies present in the gas phase, but they can select any particular one and watch its dissociation with increasing instrument voltage. In effect, the group is able to determine the relative stability of different aggregates and rapidly construct a structure-stability relationship for the gas phase."
Karen L. Wooley, chemistry professor at Washington University in St. Louis, remarks that the work is of the highest level of novelty, significance, and elegance.
"By observation and analysis of the individual host-guest complexes, this work demonstrates quantitatively the selectivity that can be achieved for individual guest dissociation events," she says. "Such advances in the fundamental understanding of these systems will accelerate the design, development, and study of new and enhanced materials that exhibit selective association/dissociation events and complex host-guest behaviors."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter