Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Science Concentrates
October 3, 2005
| A version of this story appeared in
Volume 83, Issue 40
Mesoporous germanate
Within their regular nanoscale holes, mesoporous materi- als offer excellent accommodations for chemical processes such as catalysis and adsorption. Xiaodong Zou of Sweden's Stockholm University and coworkers have prepared a novel inorganic mesoporous material that promises to broaden the scope of these structures (Nature 2005, 437, 716). The new material (shown) is made of Ge10O24(OH)3 clusters linked through Ge-O-Ge bonds. Zou's germanate structure is both mesoporous and crystalline, combining the regular structure of a smaller pored material with large pores that can be used in catalysis. This combination of properties is rare in inorganic mesoporous materials, most of which are amorphous and therefore lack the shape selectivity of crystalline materials. The material has the largest cell volume of any inorganic substance, according to the report. Pore space accounts for more than half of the germanate's total volume. Zou's group also prepared a chiral version of the mesoporous germanate, which could offer sites for stereoselective reactions.
New route to dichlorocarbene
The reactive intermediatedichlorocarbene (CCl2) can be photolytically generated from a number of precursors, but these procedures also generate aromatic by-products that can interfere with spectroscopic studies. Now, chemists at Rutgers University in New Brunswick, N.J., have found a way to prepare a nitrogenous precursor to CCl2, opening the way for detailed spectroscopic study of this carbene. The new precursor is dichlorodiazirine (shown). It was synthesized by treating 3-p-nitrophenoxy-3-chlorodiazirine with excess chloride ion, resulting in substitution of the p-nitrophenoxy group by chloride. Dichlorodiazirine is moderately stable at 25 °C in the dark and can be photolyzed cleanly to CCl2 and N2. "This preparation ends a 40-year search for a spectroscopically user-friendly precursor for CCl2," says professor Robert A. Moss, who reports the findings with coworkers Gaosheng Chu and Ronald R. Sauers (J. Am. Chem. Soc. 2005, 127, 14206). "Since the article was submitted," Moss adds, “we have obtained the first solution UV spectrum of this archetypal carbene."
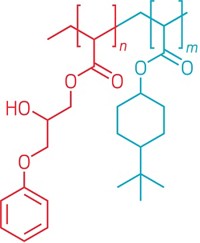
Tethered drug protects implants
A method for bonding an antibiotic covalently to titanium as a way to prevent infections on titanium orthopedic implants has been devised by Eric Wickstrom and coworkers at Jefferson Medical College of Thomas Jefferson University, Philadelphia (Chem. Biol. 2005, 12, 1041). Infections are one of the major causes of implant failure. Currently, antibiotics are administered locally around implants-in bone cement, beads, or a noncovalent coating, for example. But the activity of such antibiotics can be short-lived. The team used an ethylene glycol tether to covalently bond the antibiotic vancomycin to aminopropylated titanium. In vitro tests showed that the titanium-bound antibiotic inhibited bacterial proliferation and biofilm formation. Because the link is covalent, the antibiotic's action is expected to be long lasting. Tests in animals and people are planned. Later, Wickstrom and coworkers hope to stimulate growth of bone into implants by attaching adhesion peptides to implant surfaces along with antibiotics.
Green tea, RNA, and Alzheimer's
A constituent of green tea and a fragment of RNA have shown promise in treating mouse models of Alzheimer's disease. Green tea is reputed to protect drinkers against conditions ranging from cancer to heart disease. The tea's benefits are attributed to antioxidants known as flavonoids, including (-)-epigallocatechin-3-gallate (shown). Jun Tan of the University of South Florida, Tampa, and colleagues have now found that this compound alters cleavage of amyloid precursor protein, decreasing production of amyloid- and amyloid plaques in mice (J. Neurosci. 2005, 25, 8807). Tan's team plans to check whether the flavonoid can reverse the animals' memory loss-a feat recently accomplished by means of a gene-silencing technique. Eliezer Masliah of the University of California, San Diego, and coworkers used RNA interference to reduce expression of
and amyloid plaques in mice (J. Neurosci. 2005, 25, 8807). Tan's team plans to check whether the flavonoid can reverse the animals' memory loss-a feat recently accomplished by means of a gene-silencing technique. Eliezer Masliah of the University of California, San Diego, and coworkers used RNA interference to reduce expression of  -secretase (Nat. Neurosci. 2005, 8, 1343). Excessive activity of this enzyme, which is involved in cleaving amyloid precursor protein to create amyloid-
-secretase (Nat. Neurosci. 2005, 8, 1343). Excessive activity of this enzyme, which is involved in cleaving amyloid precursor protein to create amyloid- , is associated with Alzheimer's disease.
, is associated with Alzheimer's disease.
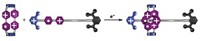
Molecules take a walk
Scientists have designed a molecule that "walks" across a surface in a straight line by putting one bond in front of the other. Such purposeful control of a molecule's motion is vital for advancing fields such as molecular self-assembly, molecular machines, and computing. The electrically charged tip of a scanning tunneling microscope lures a single 9,10-dithioanthracene molecule (shown) with thiol "legs" (purple) across a copper surface (yellow spheres), report Ludwig Bartels of the University of California, Riverside, and coworkers in an upcoming issue of Physical Review Letters. As the "body" of the molecule pivots forward, one thiol bonds to the surface while the other detaches. Previous studies have examined molecular diffusion on surfaces with step edges or anisotropic surfaces, which help keep the molecule moving in one direction. The new research was done on an isotropic surface, where all directions look the same to the molecule.






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter