Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Iron-sulfur Core Assembled
Complex mimics hydrogenase's core, suggesting fuel-cell applications
by Stu Borman
February 14, 2005
| A version of this story appeared in
Volume 83, Issue 7
INORGANIC SYNTHESIS
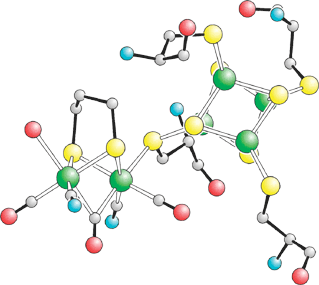
The long-sought synthesis of an inorganic complex very similar to the catalytic iron-sulfur core of bacterial hydrogenase suggests that similar synthetic systems could be developed for reversible hydrogen fuel cells that would not require expensive and rare platinum catalysts.
Hydrogenases are enzymes that enable bacteria to evolve or take up hydrogen and use it as a metabolic oxidizing or reducing agent. The new complex accelerates the same reaction as hydrogenase, although less efficiently. It was synthesized by biological chemistry professor Christopher J. Pickett of John Innes Centre, Norwich, England, and collaborators there, at Pacific Northwest National Laboratory, and at the University of Milan-Bicocca, in Italy [Nature, 433, 610 (2005)].
This goal "has been a long-standing challenge for inorganic synthetic chemists," writes chemistry professor Marcetta Y. Darensbourg of Texas A&M University in a Nature commentary. The synthetic structure "points toward a next generation of bio-inspired catalysts."
"It's a significant piece of work," comments associate professor of biochemistry John W. Peters of Montana State University, Bozeman. "This type of asymmetric bridged-metal assembly is a really difficult synthetic target. Getting something that so closely mimics the hydrogenase core will be invaluable in dissecting some intricacies that are difficult to probe in the enzyme."
The new molecule "is a real tour de force and very nice chemistry indeed," adds crystallographer Juan Carlos Fontecilla-Camps of the Institute of Structural Biology, Grenoble, France.
In hydrogenase, the core (H-cluster) consists of two components-- an Fe–Fe unit and a larger 4Fe4S cluster--that are joined through a sulfur atom on one of the enzyme's cysteine residues. The synthesis involved the creation and joining of the two components, which is very difficult. "Chemists have never before tried this build-up approach with such precise mimics of the two subsites," Darensbourg tells C&EN.
Pickett and coworkers achieved a configuration similar to that in the enzyme by activating the Fe–Fe unit with a thioacetyl group and protecting the 4Fe4S cluster with a large bowl-shaped ligand. The ligand left only one of the cluster's Fe atoms free to react, enabling formation of a correctly joined enzymelike assembly. The researchers then showed that the synthetic cluster catalyzes reduction of H+ to H2--currently with poor efficiency, but they hope this can eventually be improved.
"In addition to advancing our understanding of the natural biological system, the availability of an active, free-standing analog of the H-cluster may enable us to develop useful electrocatalytic materials for application in, for example, reversible hydrogen fuel cells," the researchers write. "Platinum is currently the preferred electrocatalyst for such applications but is expensive, limited in availability, and, in the long term, unsustainable." Reports on the hydrogen economy and fuel cells published in 2003 by the U.S. Department of Energy and the U.K. Department of Transport emphasized the long-term need to replace platinum or minimize its use in hydrogen-fuel-cell systems.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter