Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Elusive N2, CO complexes made
May 1, 2006
| A version of this story appeared in
Volume 84, Issue 18
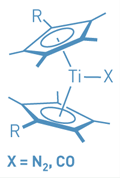
Bis(cyclopentadienyl) titanium sandwich compounds (titanocenes) have intrigued chemists because their reactivity makes them potentially useful for dinitrogen activation. But the compounds also have perplexed chemists because the reactivity, which can lead to titanium dimer formation, has hindered the study of the monomeric complexes. Introducing bulky and electron-withdrawing silyl substituents onto the cyclopentadienyl rings has helped. This strategy has now led Tamara E. Hanna, Emil Lobkovsky, and Paul J. Chirik of Cornell University to prepare the first isolable monomeric titanocenes with one N2 or CO ligand (one shown, R = Si[CH3]2C6H5), as well as disubstituted titanocenes containing N2, CO, or both ligands (J. Am. Chem. Soc. 2006, 128, 6018). Chirik's group previously prepared disubstituted N2 titanocenes in which the cyclopentadienyl R substituent was Si(CH3)3 or branched alkyl groups. It turns out that the slightly bulkier Si(CH3)2C6H5 groups are a perfect match to stabilize the monosubstituted complexes. The results provide new insight into how N2 coordination to transition metals can be controlled by careful manipulation of cyclopentadienyl substituents, the team notes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter