Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Triplet diphenylcarbene has 24-hour staying power
January 9, 2006
| A version of this story appeared in
Volume 84, Issue 2
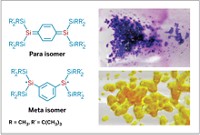
A triplet diphenylcarbene with unprecedented stability has been reported by chemists at Japan's Mie University (J. Am. Chem. Soc., published online Dec. 31, 2005, dx.doi.org/10.1021/ja056575j). Katsuyuki Hirai, Hideo Tomioka, and coworkers created an organic diradical with unpaired electrons that can survive 24 hours in solution by adding large yet unreactive substituents to the molecule's ortho positions. Hirai and Tomioka's group characterized the triplet diphenylcarbene (shown, Ph = phenyl) and found the molecule to be "at least two orders of magnitude less reactive than the most stable triplet diphenylcarbene thus far known." After a day in solution at room temperature, traces of the triplet carbene were still detectable spectroscopically. The researchers attribute the molecule's remarkable stability to a perpendicular arrangement of the CF3 groups. They suspect this orientation shields the carbene center. The Mie group also prepared an unsymmetrical positional isomer of the molecule with like substituents on the same ring. While less stable than its symmetrical isomer, this carbene is nevertheless more stable than previously reported diphenylcarbenes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter