Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Zeolite-Like Frameworks Have Large Cavities
Anionic, porous materials may be useful for trapping large molecules and cations
by Michael Freemantle
February 9, 2006
A process for synthesizing metal-organic frameworks offers the potential for introducing tunable organic functionalities into large zeolite-like cavities, according to Mohamed Eddaoudi of the University of South Florida and coworkers (Chem. Commun., published online Feb. 2, dx.doi.org/10.1039/b600188m).
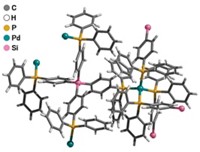
The team used a molecular building block approach to construct two novel zeolite-like metal-organic frameworks (ZMOFs) by bridging tetrahedral building units containing indium(III) ions (green) with doubly deprotonated 4,5-imidazoledicarboxylic acid linkers in the presence of a structure-directing agent such as protonated imidazole. One of the frameworks (shown, cavity is yellow)—called sod-ZMOF—has a sodalite zeolite-type topology with each In3+ ion coordinated to four nitrogen atoms (blue) and two oxygen atoms (red). The other framework, rho-ZMOF, has the zeolite rho-type topology. Its cavities are "extra-large" compared with those of sod-ZMOF and zeolite analogs.
ZMOFs are anionic, have accessible multidimensional channels and permanent porosity, and exhibit facile ionic exchange capacity. The large cavities and negative charges of the frameworks offer "great potential to explore applications involving the inclusion or encapsulation of large molecules and cations," Eddaoudi says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter