Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Groups Petition FDA On Nanomaterials
Agency is asked to devise improved product testing or face lawsuit
by David J. Hanson
May 18, 2006
A coalition of eight consumer and environmental organizations has petitioned the Food & Drug Administration to address what they call the human health and environmental risks of untested and unlabeled nanomaterials in consumer products.
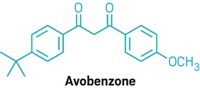
Led by the Washington, D.C.-based International Center for Technology Assessment, the groups are concerned that FDA has taken no steps to develop health and safety tests for products containing nanomaterials despite the groups'assertion that these materials have "fundamentally different properties from their bulk material counterparts." Part of the petition specifically seeks FDA action on titanium dioxide and zinc oxide nanomaterials used in sunscreens.
The 80-page petition includes information related to nanomaterial toxicity, including previous reports that nanoparticles have been found to penetrate cells and to trigger inflammatory and immune responses. It also cites the recent case in Germany where a cleaning product was removed from the market after several persons were hospitalized with lung problems that may have been associated with using the cleaner (C&EN, April 17, page 10).
Sunscreens were highlighted because "they are the poster child of FDA's gross failure to adequately protect the public's health and safety," said ICTA attorney George Kimbrell. These products are unlabeled, untested, and placed repeatedly directly on human skin, Kimbrell continued.
FDA does not comment on any pending legal challenges and has six months to respond to the petition, after which the groups can file a lawsuit on the matter.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter