Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Enzyme oxidizes single-handedly
May 21, 2007
| A version of this story appeared in
Volume 85, Issue 21
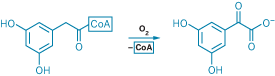
Enzymes that catalyze oxidation reactions typically rely on metal or organic cofactors to activate molecular oxygen. A handful of unusual oxygenase enzymes activate O2 single-handedly, however, and Boston College chemists now think they know how such enzymes manage this feat. Steven D. Bruner and colleagues present a 2.75-Å X-ray structure of DpgC, a cofactor-free dioxygenase that catalyzes a key step in the biosynthesis of an amino acid required to assemble the antibiotic vancomycin (Nature 2007, 447, 342). DpgC incorporates both oxygen atoms of a single O2 molecule into its substrate and releases coenzyme A (CoA), yielding a precursor to the necessary amino acid, as shown. In the crystal structure, Bruner's team discerned what they believe to be O2 tucked into a hydrophobic corner of the enzyme's active site near the substrate oxidation site. On the basis of the structure, the researchers suggest an unusual mechanism in which the substrate provides the reducing power to activate O2 to perform the oxidation.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter