Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Industry On The Mend
Drug companies' first-quarter results show early signs of healthier product portfolios
by Lisa M. Jarvis
May 21, 2007
| A version of this story appeared in
Volume 85, Issue 21
AFTER EXPERIENCING virtually flat earnings and modest sales growth in the fourth quarter of last year, pharmaceutical companies showed signs of improvement in the first quarter of 2007. The U.S. companies with the rockiest track records in 2006 are starting to stabilize in 2007, benefiting from growing sales of newer products and restructuring efforts.
At Merck, newer products are largely balancing the loss of patent protection on the firm's blockbuster cholesterol drug Zocor and pressure on sales of its osteoporosis treatment Fosamax. Overall sales were up 6.6% over the year-ago quarter to $5.8 billion, while earnings totaled $1.7 billion, a 12.1% improvement.
Zocor, which once brought in more than $4 billion annually, lost patent protection last June. First-quarter sales of the drug plummeted 76% to $258 million. Similarly affected by the influx of generics is Fosamax; sales dipped 2% to $742 million, as generic products were launched in several markets outside the U.S.
Newer products buoying Merck's results include two vaccines, a diabetes drug, and two cholesterol-lowering treatments.
The controversial cervical cancer vaccine Gardasil, approved last June, raked in $365 million in the quarter. Merck says the figure includes the first round of vaccine purchases by a number of states. Rotateq, a vaccine approved last year to guard children against rotavirus disease, brought in $85 million.
The type 2 diabetes drug Januvia, launched in October, racked up $87 million in sales in the first quarter. In the future, Merck's diabetes franchise is expected to be strengthened by the recent approval of Janumet, an oral therapy that combines Januvia with metformin, the most commonly used drug in the U.S. to treat type 2 diabetes.
Merck's cholesterol franchise also excelled in the quarter. Combined sales of Zetia and Vytorin, which Merck markets with Schering-Plough, surged 47% to $1.2??billion.
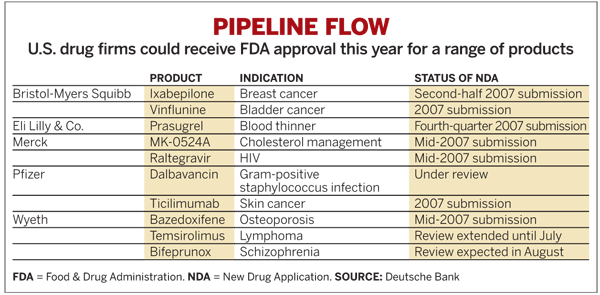
The momentum for Merck's new product portfolio is expected to pick up this year. Barbara Ryan, a Deutsche Bank stock analyst who calls Merck's new offerings "superior to its peers," expects Januvia and Gardasil to achieve peak sales of $2 billion each. Merck also is poised to file for approval for raltegravir, a first-in-class integrase inhibitor for the treatment of HIV, this quarter.
Meanwhile, Pfizer is also starting to look healthier, although it is still heavily reliant on its anticholesterol drug Lipitor. Sales grew as the firm's newer products gained market acceptance, while earnings benefited from a leaner organization after the early phases of a major restructuring. Pfizer's first-quarter profits were up 10.4% to $4.8 billion, based on a 6.2% rise in sales to $12.5 billion.
Though overall prescriptions were down for Pfizer's most important product, Lipitor, sales of the drug still increased 8% to $3.4 billion in the period. The improvement was largely due to price increases and lower rebates, which offset the impact of Lipitor patients switching to generic versions of Merck's Zocor.
Several of Pfizer's new products were also strong performers. First-quarter sales of the nerve pain treatment Lyrica more than doubled to $395 million, while the smoking-cessation drug Chantix, approved a year ago, brought in $162 million. Sales of the cancer drug Sutent grew to $102 million, compared with $16 million in the first quarter of 2006.
More than a year after its approval in the U.S., however, Exubera continues to be a major disappointment for Pfizer. Sales of the inhaled insulin product were essentially nil in the quarter, and analysts are wondering just how long Pfizer will stick with it. According to Jami Rubin, a stock analyst at Morgan Stanley, Pfizer will have roughly $1.5 billion in Exubera inventory by the end of the year. "We continue to question how the mounting inventory build can be reconciled to the dismal prescription performance," she says.
Going forward, the flow of new products is expected to be steady. Pfizer is expected to unveil 49 abstracts spanning 10 clinical programs next month at the annual meeting of the American Society of Clinical Oncology, points out Bank of America analyst Chris Schott.
In addition, Pfizer highlighted actions it is taking to improve productivity. In addition to restructuring its R&D activities around therapeutic areas, the company says it has "embarked on a concerted talent-retention and recruitment strategy." And in addition to its in-licensing efforts, which have resulted in more than 30 deals in the past 15 months, Pfizer recently established an incubator at its La Jolla, Calif., site that aims to draw in academic innovators.
Bristol-Myers Squibb continues the trend of strengthening first-quarter results. It appears to have largely rebounded from the combined impact of generic competition to the blood thinner Plavix and the cholesterol drug Pravachol. First-quarter sales totaled $4.5 billion, a 4.3% decline; however, earnings improved 16.8% to $744 million.
Sales of Plavix bounced back strongly in the first quarter compared with the second half of 2006, indicating that distributors are finally running out of generic product that entered the market prematurely. The Canadian drug firm Apotex shipped a boatload of its generic version of the blood thinner in August, before a judge issued a preliminary injunction blocking the sale until the legal dispute between the two firms is settled (C&EN, Sept. 11, 2006, page 10).
Plavix, which is comarketed with Sanofi-Aventis, brought in $986 million for BMS in the quarter, a 5% decline. Sales would have been $300 million to $350 million higher without the leftover generic product from Apotex on the market, BMS estimates. The company believes some generic impact could still be evident in the second quarter, but prescription data from consulting firm IMS Health indicate the supply is nearly exhausted, says Deutsche Bank's Ryan.
Meanwhile, other drugs in the BMS portfolio posted solid results in the quarter. Sales of the schizophrenia drug Abilify surged 29% to $366 million, and the protease inhibitor Reyataz saw sales increase 27% to $263 million. The hypertension drug Avapro, which BMS also comarkets with Sanofi-Aventis, brought in $270 million, a 16% improvement.
Sales for BMS's newer drugs were more modest. The rheumatoid arthritis treatment Orencia, launched in the first quarter of 2006, brought in $41 million, while the cancer treatment Sprycel, launched last July, had sales of $21 million.
THE RELATIVELY STRONG quarter was overshadowed by speculation about the future of BMS. James M. Cornelius, who has served as interim chief executive officer since Peter R. Dolan was ousted in September, agreed to take the CEO role permanently. Cornelius shepherded the medical device company Guidant through its takeover by Boston Scientific, and many analysts believe the BMS board brought him in to do the same for BMS.
Yet, another key announcement by BMS, made on the same day as with the earnings report, has some analysts convinced that BMS will go it alone. The firm entered a $1 billion pact with Pfizer to codevelop BMS's blood thinner apixaban, which is currently in Phase III trials. "Partnering of apixaban makes the rationale for a merger with Sanofi-Aventis less obvious," Morgan Stanley's Rubin says.
While some U.S. pharma majors appear to be on the mend, at least two of their European counterparts, Novartis and AstraZeneca, saw continued strong growth.
At Novartis, first-quarter profits improved 11.0% to $2.2 billion, based on an 18.3% increase in sales to $9.8 billion. Driving the results are a 47% rise in vaccine sales and strong sales growth for the blood pressure drug Diovan and the cancer drug Gleevec, which brought in $1.2 billion and $674 million, respectively.
AstraZeneca reported a 12.7% increase in sales to $7.0 billion and a 10.9% rise in profits to $2.3 billion. The company benefited from robust sales of its cholesterol drug Crestor, which brought in $628 million, and the strong performance of its respiratory and cancer portfolios.
However, GlaxoSmithKline posted profits of $4.2 billion, down 1.0%, while sales decreased 3.8% to $10.9 billion. Though GSK's results weren't as good as those of its European competitors, the company expects to launch five new products this year that could catalyze growth in the future.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter