Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
When Organics Fail, Try Water
Ladder polyethers form readily from epoxides in water
by Bethany Halford
September 3, 2007
| A version of this story appeared in
Volume 85, Issue 36

BY REPLACING organic solvents with water, chemists have found a simple route to selectively make ladder polyethers, including the building blocks of notorious toxins, via an epoxide-opening cascade reaction (Science 2007, 317, 1189).
Complex ladder polyether natural products, so named for their runglike structure, are the active toxins found in the harmful algal blooms known as red tides. Red tides cause devastating ecological damage, so scientists hope that by studying this water-promoted cascade reaction, they will gain a better understanding of how and why these toxins form.
The finding also offers a solution to what had been a long-standing puzzle for synthetic chemists. Twenty years ago, Koji Nakanishi, a chemistry professor at Columbia University, suggested that ladder polyether natural products arose biosynthetically from an elaborate cascade of epoxide-opening reactions that zip up the polyether structure.
"The Nakanishi hypothesis is very intellectually appealing," says Timothy F. Jamison, a Massachusetts Institute of Technology chemistry professor who spearheaded the current study. "It accounts for so much complexity in a very simple way."
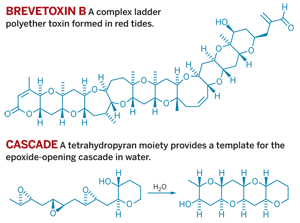
The only problem was that when organic chemists attempted this cascade in the lab, they found that Nakanishi's pathway was kinetically disfavored. Rather than forming the toxin's characteristic fused tetrahydropyrans, the cascade reaction tended to open the epoxide from the opposite side, generating a chain of spiro-tetrahydrofurans. To synthesize ladder polyethers via a cascade reaction, chemists had to use onerous directing groups—until now.
Inspired by the elegance of Nakanishi's proposed cascade, Jamison and graduate student Ivan Vilotijevic sought a transformation that would prove the hypothesis and provide a simplified route to the ladder polyether structure. They reasoned that a tetrahydropyran within the molecule could provide a template for the desired reaction.
Then, after trying hundreds of reaction conditions, they discovered, much to their surprise, that while many organic solvents usually favored the undesired reaction pathway, water proved to be an excellent solvent for the desired epoxide-opening cascade.
A lot of organic chemists shy away from using water as a solvent, Jamison says, but its effects in this case were so profound that he and Vilotijevic couldn't help but take notice.
"Many synthetic groups labored for years in attempts to mimic Nakanishi's ingenious polyepoxide-zip-biosynthesis hypothesis," says K. Barry Sharpless, a chemistry professor at Scripps Research Institute. "Now, Vilotijevic and Jamison have provided the marvelous insight that the desired cascade cyclizations work after all, provided they occur in water at neutral pH."
"This is a nice piece of work indeed," adds K. C. Nicolaou, also a chemistry professor at Scripps. "The cascade reactions reported by the Jamison group are amazing and shine new light on the possible biosynthesis and chemical synthesis of the polyether marine toxins, of which there are many. And once again, one marvels at the special water effects on accelerating and directing chemical reactions." He also notes that developing reactions in water is a primary means of furthering green chemistry goals.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter