Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Protecting Our Cultural Heritage
National Park Service program funds research to develop and apply technology for cultural preservation
by Celia Henry Arnaud
December 10, 2007
| A version of this story appeared in
Volume 85, Issue 50

THE NATIONAL Park Service has a well-kept, if unintended, secret: Because of its responsibility for many historic and cultural resources, the park service is in the business of historic preservation.
To help carry out that mission, the National Center for Preservation Technology & Training (NCPTT), established by an act of Congress in 1992 on the campus of Northwestern State University in Natchitoches, La., supports research to develop and apply science and technology to historic preservation. Some of that research is performed at NCPTT by its staff of 15. The rest is done elsewhere by scientists, engineers, and conservators funded through NCPTT's grants program. The program, which awarded its first one-year grants in 1994, funds the development or application of new technologies in the areas of architecture, archaeology, historic landscapes, and materials conservation.
Successful grant applications fulfill several criteria, according to Mary F. Striegel, chief of materials research at NCPTT. The projects meet a national need, create new technology or transfer existing technology from other fields to preservation, disseminate the results, and do this all in a cost-effective manner.
NCPTT's research priorities include the protection of cultural resources from vandalism, looting, terrorism, and natural disasters, Striegel says. The center wants to monitor and evaluate preservation treatments, determine environmental effects on cultural resources, and document and preserve cultural landscapes. A special initiative involves the preservation of storm-damaged cultural resources.
A session at the Eastern Analytical Symposium, held last month in Somerset, N.J., celebrated the center and some of the success stories from its grants program.
Many of NCPTT's grantees focus on physical and engineering methods for architecture and archaeology, but some projects develop chemical and biological methods for the preservation of materials and cultural heritage objects. In some cases, those methods must reverse the ill effects of earlier treatments.
For example, in the past, many objects in museum and private collections were treated with pesticides containing mercury and arsenic. Health concerns dictate that these toxic metals be removed, but without damaging the artifacts. Such remediation is particularly important for items such as Native American artifacts that are being returned to the tribes that are their rightful owners who may wish to use the artifacts in rituals.
One such remediation method for heavy metal contamination funded by NCPTT is being developed by Timberley Roane, an associate professor of biology at the University of Colorado, Denver. As an environmental microbiologist, Roane wondered whether bacteria might be able to remove mercury and arsenic from cultural objects. From her environmental work, Roane knew of the existence of mercury-resistant bacteria that reduce mercury ions to elemental mercury, which then diffuses away, decreasing the effective concentration of the toxic metal around the bacteria.
MANY SUCH BACTERIA are found in runoff around mining operations, but Roane wanted to see if she could find effective bacteria within the milder conditions of the museum environment. She sampled objects in the Arizona State Museum for bacteria that she then cultured and selected for mercury resistance. Her search yielded a species from a genus of common soil bacteria (Arthrobacter) that can remove about 30% of mercury contamination. Such performance, however, is outgunned by Cupriavidus metallidurans, a bacterium isolated from zinc mine tailings that removes 60–80% of the mercury.

C. metallidurans is also less likely to damage organic material in artifacts because it is an autotrophic bacterium that can make its own food from inorganic sources. Although Arthrobacter species don't seem to metabolize complex organic compounds such as fatty acids and cellulose, they do eat simple sugars, amino acids, and organic acids. Whether such a limited diet will damage objects has yet to be determined.
NCPTT is also funding chemical methods to remove heavy metals from cultural artifacts. In the symposium, Peggi Cross, now at Spectra-Physics in Tucson, Ariz., presented the work she did while a graduate student in materials science and engineering at the University of Arizona. She used a solution of dihydrolipoic acid, the reduced form of the natural chelator α-lipoic acid, to remove mercury from objects. Mercury reacts with α-lipoic acid and arsenic reacts with dihydrolipoic acid to form metal organic compounds that can be removed from materials.
In her work, Cross showed she could remove arsenic from filter paper, cotton, wool, and feathers with dihydrolipoic acid. Her method also removed mercury from materials that do not contain sulfur. For tribes that want to rebury artifacts, the lipoic acid treatment may work as a coating to prevent metals from getting into the environment, Cross said.
NCPTT is also interested in funding research to protect more traditional works of art such as paintings and sculptures. In one such project, Barbara Berrie, a senior conservation scientist at the National Gallery of Art in Washington, D.C., is using gels for cleaning paintings and sculptures.
Gels are attractive materials for conservation applications because, compared with liquid treatments, there's less solvent to evaporate or penetrate into the object. Gels also allow greater spatial control of cleaning. Despite these good features, the use of gels has been limited because they are usually difficult to make, manipulate, and remove after application.
Rheoreversible gels, which are stiff under some conditions and liquid under others, could make gels easier to use. At the NCPTT symposium, Berrie described three classes of reversible gels she is using that were developed in the lab of her collaborator, chemistry professor Richard Weiss of Georgetown University: poly(allylamine) (PAA) with CO2 and poly(ethyleneimine) (PEI) with CO2, both of which are pH reversible; and poly(vinylalcohol) (PVA) with borate, which is thermally reversible.
The gels are easy enough to use that even Berrie, who does not usually work directly with artwork, has been able to safely clean paintings with them, but none of those works were part of the National Gallery's collection. Berrie finds the PEI gels easier to make and work with than the PAA gels, which require a lot of preparation. The PVA gel is not reversible enough to use on paintings, she says, noting that it has been used to clean limestone statues.
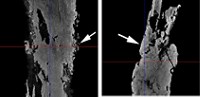
NCPTT IS ALSO interested in new methods to treat waterlogged wooden archaeological objects. Any method for treating wet wood must include a method for drying the wood. Eric Schindelholz, an objects conservator at the National Park Service's Harpers Ferry Center in West Virginia, compared conventional methods of drying waterlogged wood with a new supercritical fluid drying method.
Waterlogged wood is prone to structural collapse because the cells in the wood shrink as it dries out, due to capillary stress imposed by evaporating water. Conservators need methods for drying waterlogged wood that minimize that capillary stress. Current methods include slow air-drying and solvent replacement, diffusing inert material into the wood, and freeze-drying the wood. The most commonly used method involves diffusing polyethylene glycol into the wood followed by freeze-drying. The problem is that the treatment is irreversible and takes a long time.
In the supercritical drying technique, the water in the wood is replaced with methanol, which is in turn replaced with supercritical carbon dioxide. The wood then dries in a chamber at elevated pressure and temperature. The results depend on the shape of the wood and whether it is in the form of blocks or planks. The size of the chamber limits the objects that can be treated, and the chamber available to Schindelholz's team was only 250 mL—only big enough for something the size of a child's building block.
Schindelholz compared the effects of supercritical drying and freeze-drying on objects made of pine and walnut. Walnut responded well to both methods, but cracks appeared on the pine's surface in supercritical drying, with shrinkage more pronounced in highly degraded sections of the wood. He and his coworkers still need to test other types of wood and to vary the conditions of supercritical drying, Schindelholz said. They will also need to test the method in larger chambers to make sure it is applicable to real-world artifacts, he said.
These are only a few of the projects that NCPTT has funded over the years. The number of grants that NCPTT can award, however, has steadily dropped since the first set in 1994. NCPTT has never received a funding increase, and as the cost of running the center increases, the money available for external grants decreases, Striegel says. Nevertheless, NCPTT continues to bring technology to the field of cultural preservation.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter