Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Faster, More Sensitive 2-D NMR
Technique combines ultrafast NMR with hyperpolarization
by Stu Borman
May 7, 2007

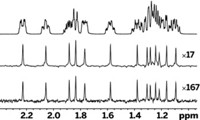
Researchers have devised a way to obtain two-dimensional nuclear magnetic resonance (2-D NMR) spectra with unprecedented sensitivity and speed.
In applications ranging from organic chemistry and drug research to structural biology, 2-D NMR spectroscopy is particularly suited for analyzing complex molecular systems. But it's slow and not very sensitive. Lucio Frydman, professor of chemistry at Weizmann Institute of Science, Rehovot, Israel, and application scientist Damir Blazina of Oxford Instruments Molecular Biotools, Oxford, England, now have developed a technique that addresses these problems (Nat. Phys., DOI: 10.1038/nphys597).
The technique combines two previous methods: ultrafast multidimensional NMR and ex situ hyperpolarized dynamic nuclear polarization (DNP). The former, developed in 2002 by Frydman's group, uses magnetic field gradients and temporal and spatial encoding of NMR signals to acquire each multidimensional NMR spectrum rapidly in a single scan. DNP, developed the following year by NMR spectroscopist Klaes Golman and coworkers at Amersham Health Research & Development, Malmö, Sweden, boosts sensitivity by hyperpolarizing nuclei, aligning a greater than usual proportion of them with respect to an external magnetic field.
It wasn't clear that the two techniques would be compatible, but Frydman and Blazina got them to play nicely together. They call their combined technique hyperpolarized ultrafast multidimensional NMR spectroscopy, but so far they have demonstrated it only for 2-D NMR.
The merging of techniques makes it possible "to collect 2-D NMR data within a fraction of a second, instead of the minutes to hours normally required for conventional 2-D NMR acquisitions," Frydman says. And it makes it possible to analyze samples with submicromolar concentrations, whereas millimolar concentration limits are more typical of conventional NMR, he says.
Frydman points out that it takes time to set up the new procedure. The hyperpolarization part of the process is slow and time-consuming, which reduces the technique's speed advantage somewhat, he adds. "There's a need to shorten the hyperpolarization time," he says.
The main advantage of the new approach right now is the ability "to do 2-D NMR on very dilute samples—even if we have to wait a while before typing 'go' on the spectrometer," Frydman says.
Asked to comment on the work, NMR specialist Bernhard Blümich of RWTH Aachen University, in Germany, says, "It's certainly a breakthrough, and the combination of techniques is highly innovative."
The new approach doesn't have the generality of conventional NMR because hyperpolarization "works well for some molecules and not so well for others," Blümich says. And he notes that the long setup time of hyperpolarization experiments is a problem. But the work is still at a basic research stage, and the new technique could be improved, he says.
The technique might be used to study catalysis on surfaces, an application for which conventional NMR has insufficient sensitivity, Blümich says. It also could be useful to scientists who want to study dynamic phenomena and intermediate species on very short time scales. "The sense of pushing the whole field of NMR into a new, exciting direction is something quite remarkable," he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter