Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Steric Zippers Play Key Role In Amyloid Fibrils
Study indicates that Alzheimer's, type 2 diabetes, and other degenerative diseases are closely related at the molecular level
by Stu Borman
May 3, 2007
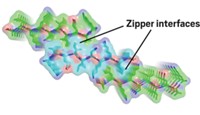
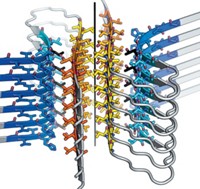
Two years ago, David Eisenberg of the University of California, Los Angeles, and a collaborative international group obtained the first atomic structure of an amyloid fibril formed from a yeast prion protein and found that an interdigitated β-sheet structure called a steric zipper was essential to fibril formation (C&EN, June 13, 2005, page 9).
Now, the same group has confirmed that steric zippers are a common structural feature in such aggregates by determining the atomic structures of microcrystals formed by 30 peptides associated with Alzheimer's disease, Parkinson's disease, type 2 diabetes, Creutzfeldt-Jakob disease, and other amyloid-related conditions (Nature, DOI: 10.1038/nature05695).
The researchers used specialized techniques, including synchrotron radiation microcrystallography, to obtain structures of the amyloid microcrystals, which otherwise would be too small to permit structural analysis. The work suggests that "amyloid diseases are similar not only on the fibril level, but also on the molecular level," the researchers write.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter