Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Copper-Free Click Chemistry
Reagent for labeling biomolecules eliminates need for toxic metal catalyst
by Celia Henry Arnaud
October 18, 2007
A new reagent developed by chemistry professor Carolyn R. Bertozzi and coworkers at the University of California, Berkeley, eliminates the toxicity usually associated with a rapid and irreversible reaction strategy commonly known as "click chemistry" (Proc. Natl. Acad. Sci. USA, DOI: 10.1072/pnas.0707090104). This tweak to remove copper catalysts makes the reaction, azide-alkyne cycloaddition, biologically friendly and thus useful for labeling biomolecules in cells.

The reagent helps Bertozzi and her team study dynamic biochemical processes that are otherwise difficult to follow in real time. Bertozzi is particularly interested in studying glycosylation, the addition of sugar molecules to proteins. The reaction is tough to track because the sugar molecules, or glycans, are continuously recycled. "Most imaging of carbohydrates uses fixed systems," says Jeremy M. Baskin, a grad student in Bertozzi's lab and lead author of the report. "You can take a snapshot, but you can???t make the equivalent of a movie."
Azides make a handy tag for labeling biomolecules. They don't react with other molecules in the system, and they can be added to a range of biomolecules, including sugars, lipids, and proteins.
Unfortunately, the two reactions most commonly used to affix fluorescent or other labels to azide-tagged biomolecules have limitations. The Staudinger ligation, which forms an amide bond between the azide and an ester-derivatized phosphine, is too slow. Azide-alkyne cycloaddition is much faster, but the conventional copper catalysts required are toxic to living systems.
Bertozzi's team has designed a reagent that eliminates those copper catalysts. "This copper-free chemistry is really designed for applications that require this extra burden of nontoxicity," Baskin says. "I see it as one reaction in a family of click chemistries."
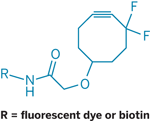
The UC Berkeley researchers speed up the reaction even without a catalyst by using a difluorinated cyclooctyne instead of the usual terminal alkyne. The ring strain and the electron-withdrawing difluoro group activate the alkyne for copper-free click chemistry.
Bertozzi and coworkers use the reagent to attach fluorescent labels to cells with azide-containing sialic acid in their surface glycans. The team studied the dynamics of glycan trafficking over the course of 24 hours with no indication that the reaction or the labels perturb the process.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter