Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Modified Serine Controls Protein Phosphorylation
Light releases serine protecting group, permitting researchers to regulate cell-signaling mechanism
by Celia Henry Arnaud
October 31, 2007
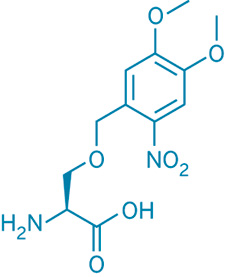
Protein phosphorylation, which is a major mechanism of cell signaling, can occur only on certain amino acids, including serine. Peter G. Schultz and coworkers at Scripps Research Institute report that they can control protein phosphorylation on serine residues by genetically incorporating a photoprotected serine analog into proteins (Nat. Chem. Biol., DOI: 10.1038/nchembio.2007.44).
Phosphorylation can't occur at the protected serine residues. But shining blue light on the protein removes the 4,5-dimethoxy-2-nitrobenzyl protecting group, thereby exposing serine and making it available for phosphorylation.
The researchers used the serine analog to regulate phosphorylation of specific serine residues in Pho4, a yeast transcription factor that regulates genes and allows the organism to grow at different inorganic phosphate concentrations. By replacing one serine residue at a time with the photoprotected analog, the team was able to observe the effects of blocking phosphorylation at specific sites and measure dynamic responses to the phosphorylation event.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter