Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
The Shortest Metal-Metal Bond Yet
Dichromium complex sporting a quintuple Cr???Cr bond sets a new record at 1.8028 Å
by Stephen K. Ritter
November 7, 2007

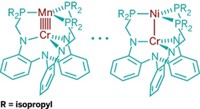
Multiple bonding between two transition-metal atoms is a constant source of fascination for inorganic chemists interested in the nitty-gritty of chemical bonding. The current upper limit in an isolable compound is the quintuple bond, which was first observed two years ago in the form of a dichromium complex.
In the latest multiple-bonding advance, graduate student Kevin A. Kreisel and chemistry professor Klaus H. Theopold of the University of Delaware and coworkers have synthesized a new type of quintuple-bonded dichromium complex, one that surpasses all known compounds for having the shortest measured metal-metal bond length (J. Am. Chem. Soc., DOI: 10.1021/ja076356t).
Until the mid-1960s, chemists largely assumed that the triple bond was the highest possible multiple bond. But in 1964, F. Albert Cotton and coworkers at Texas A&M University surprised the chemistry community with evidence that the [Re2Cl8]2??? ion contained a quadruple bond between two metal atoms. Since then, chemists have observed many quadruple-bonded transition-metal compounds.
It took another 40 years to observe a higher level of bonding; in 2005, Philip P. Power of the University of California, Davis, and coworkers extended the multiple-bond boundaries by reporting a quintuple bond in the dichromium complex RCrCrR, where R designates a terphenyl ligand with diisopropyl substituents (C&EN, Sept. 26, 2005, page 9). This bulky ligand helps to stabilize the complex and minimize the number of metal-ligand bonds, which promotes a higher degree of metal-metal bonding, Power tells C&EN.
Power notes that the two chromium(I) atoms, which have a 3d5 electron configuration, share five electron pairs in five bonding molecular orbitals. Despite this fivefold bonding interaction, the actual degree of bonding—the calculated bond order—is only 3.52 because of mixing in of antibonding character from molecular orbitals, he explains.
The Cr???Cr bond length in Power's complex is 1.8351 ??. The record at the time, however, still belonged to Cotton, who set the standard of 1.828 ?? in 1978 with a quadruple-bonded dichromium complex (Inorg. Chem. 1978, 17, 2084). Cotton's complex utilizes four bridging methoxyphenyl ligands that form a cage to restrain the chromium atoms.
Kreisel and Theopold took a page from both Cotton's and Power's notebooks for their dichromium complex: They used restrictive ligands similar to those in Cotton's complex and were able to still achieve high-order bonding similar to that of Power's complex. Kreisel actually discovered the complex by accident and later proceeded to optimize it, Theopold says, "as the team originally was not out to make Cr???Cr compounds."
In the complex, each chromium atom is coordinated to two nitrogen atoms, one each from two bridging diazadiene ligands containing diisopropylphenyl groups. The result is a tight Cr2N4 core structure. The most noticeable feature is the very short 1.8028-Å Cr–Cr bond, which is the shortest metal-metal distance reported to date for an isolable compound. Dimetal compounds with shorter bonds have been observed in gas-phase experiments.
In collaboration with Clark R. Landis of the University of Wisconsin, Madison, the researchers supported their experimental observations with calculations to confirm the bonding and electronic structure of the complex, including a bond order of 4.28. Kreisel, now a postdoc in Landis' lab, serendipitously established the fruitful collaboration during an interview visit to Wisconsin.
"It's really a very nice paper," Power says of the work by Kreisel, Theopold, and Landis. The Cr???Cr distance in the new complex "is quite interesting," he adds. "But the unique aspect, perhaps the most important observation, is clear evidence for five bonding interactions."
Power notes that his group has just completed additional studies of substituent effects in his dichromium complex. With modified ligands, the researchers have reeled in their bond length to 1.8077 Å, just shy of the new record. The calculated bond orders are above 4, Power says.
Power and Theopold recently discussed the dichromium bonding. Power says he and Theopold agree that "there's no reason to suppose that the lower limit in metal-metal bond distances has been reached. Eventually, someone will get a shorter one."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter