Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Two-Piece Imaging Probes
Fluorescent inclusion complex assembles inside cells
by Bethany Halford
December 5, 2007

By wrapping a squaraine dye in a molecular cloak, chemists at the University of Notre Dame, Indiana, have managed to shield the reactive dye compound from nucleophiles and improve its stability, thereby increasing its viability as a near-infrared fluorescent-imaging probe for cellular and other biological studies (J. Am. Chem. Soc. 2007, 129, 15054).
For a molecule to be a good near-IR fluorescent-imaging probe, it needs to have two things going for it: It must fluoresce brightly, and it has to be long-lived enough to shine through the duration of an imaging experiment. Squaraine dyes possess an intense fluorescence emission, but their electron-deficient squaric acid core makes them prone to nucleophilic attack.
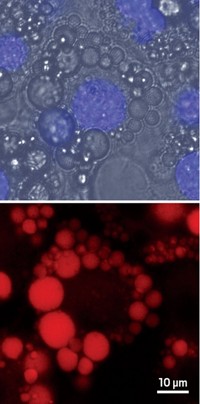
A team led by Bradley D. Smith, Jeremiah J. Gassensmith, and Easwaran Arunkumar found that by threading a squaraine dye molecule through an anthracene-containing tetralactam macrocycle, they could stave off nucleophilic attack while still preserving the molecule's fluorescence. The inclusion complex's emission is red-shifted from that of the lone squaraine, making it easy to differentiate the two-piece probe from its individual components.
Furthermore, the Notre Dame team found that the complex readily self-assembles within the complicated molecular environment of a living cell. Next the researchers plan to modify the system so that it can be used as a molecular probe for cell and whole-animal imaging.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter