Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cyclization In Close Quarters
March 24, 2008
| A version of this story appeared in
Volume 86, Issue 12
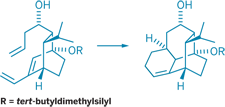
The Diels-Alder reaction, a widely used transformation in organic synthesis, forms six-membered rings from a conjugated diene and a dienophile-frequently an alkene. Usually, these two reactants must be activated by catalysts or have complementary electronic properties to participate in the reaction. Phil S. Baran, Thomas J. Maimone, and Ana-Florina Voica of Scripps Research Institute have observed a catalyst-free Diels-Alder reaction between simple, nonactivated reactants at room temperature while synthesizing an intermediate en route to vinigrol, a natural product with antihypertensive and other biological activities (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200800167). Vinigrol has a unique three-ring skeleton, which the team formed by strategically breaking a bond in a four-ring intermediate. The Diels-Alder reaction (shown) forms the four-ring intermediate from a bicyclic precursor. The team believes that the precursor's compact structure brings the reacting diene and alkene into proximity and promotes the cyclization. The rigidity of the resulting four-ring intermediate likely aids the subsequent bond-breaking step that forms vinigrol's three-ring skeleton, the authors write. They now hope to complete the synthesis of vinigrol from this skeleton.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter