Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Heparin Unveiled
Complex polysaccharide facilitates more than just blood thinning
by Jyllian Kemsley
March 24, 2008
| A version of this story appeared in
Volume 86, Issue 12
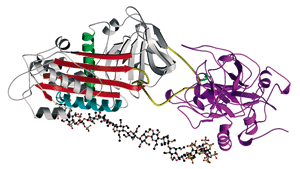
Heparin (ball and stick structure) activates antithrombin-III (gray) to bind to and inhibit thrombin (purple).
THE ANTICOAGULANT heparin is nearly ubiquitous in hospitals, where it is used to treat heart attack patients, prevent clots from forming during heart surgery and dialysis, flush intravenous lines, and coat blood collection tubes. It is so critical to U.S. medical care that when Baxter Healthcare wanted to expand a recall of heparin products earlier this year to address an increasing incidence of severe reactions to the drug, the Food & Drug Administration delayed the recall for three weeks to prevent a shortage while another supplier ramped up production.
All this raises the questions: Just what is heparin? Where does it come from? What does it do? How is it isolated?
First and foremost, heparin is a biological product. Chemically, it is a variably sulfated glycosaminoglycan polysaccharide. It is composed of repeating disaccharide units of a uronic acid 1,4-linked to a D-glucosamine.
Biosynthesis of heparin starts with a tetrasaccharide primer attached to a core protein. N-Acetyl D-glucosamine and glucuronic acid monosaccharides are alternately added by tranferase enzymes. As the polysaccharide chain grows, enzymes modify the sugars by removing acetyl groups and adding N-sulfate groups. Some of the D-glucuronic acid residues are then converted into l-iduronic units by a C-5 epimerase. Finally, the polysaccharide is O-sulfated at varying locations.
The result is a complex heteropolymer ranging in molecular weight from 3 to 40 kilodaltons; the average for commercial preparations is 12-15 kDa. Whereas DNA has four building blocks and proteins have 20, heparin theoretically has 48. "The chemical diversity is enormous," says Ganesh Venkataraman, chief scientific officer of Momenta Pharmaceuticals, which is developing a generic version of enoxaparin, a low-molecular-weight heparin (LMWH) currently marketed by Sanofi-Aventis as Lovenox. Enoxaparin is prepared by chemically cleaving the longer polysaccharide chains to smaller pieces with molecular weights of about 3 kDa.
Heparin's physiological role is that of a catalyst, facilitating protein conformation changes and protein-protein interactions. It is found in a variety of tissues but is specific to mast cells, which are involved in inflammatory responses. It is localized to the extracellular matrix, often activating a protein to interact with a receptor. The geometric structure—how the sugar backbone twists and kinks—defines the topology of the sulfate groups recognized by proteins, Venkataraman notes.
Clinically, heparin inhibits coagulation by binding to antithrombin III (AT-III), which recognizes a specific pentasaccharide sequence of the drug. AT-III undergoes a conformational change that activates it to bind and inhibit either of the enzymes thrombin or factor Xa.
By inhibiting thrombin, AT-III shuts off the formation of fibrin, the protein that polymerizes into the mesh that underlies a blood clot. Inhibiting factor Xa stops the conversion of prothrombin to thrombin. Heparin's AT-III-binding pentasaccharide sequence is all that's needed to induce factor Xa inhibition, and this is the clinical mechanism of LMWH, as well as that of the synthetic drug fondaparinux, marketed by GlaxoSmithKline as Arixtra. For AT-III inhibition of thrombin, a chain of 14–20 saccharide residues is needed, although outside of the AT-III-specific pentasaccharide the sequence can be random.
But heparin does more than just inhibit the clotting cascade. It plays a role in 37 major disease areas, notes Peter H. Seeberger, a chemistry professor at the Swiss Federal Institute of Technology, in Zurich. For example, heparin binds to fibroblast growth factors, activating them to bind to receptors and promote cell proliferation, differentiation, and angiogenesis.
Heparin also interacts with chemokines, which are glycoproteins involved in angiogenesis, breast cancer metastasis, and migration of white blood cells. In particular, heparin binds to platelet factor 4 (PF4), an interaction that is believed to compete with heparin-AT-III binding and thus allow for clotting. When heparin is used therapeutically, however, the body will sometimes generate an immune response to the heparin-PF4 complex, eventually leading to loss of platelets in a serious side effect called heparin-induced thrombocytopenia.
Used clinically as an anticoagulant since the 1930s, heparin—originally isolated from canine liver cells—is currently isolated from pig intestines. Heparin production varies by manufacturer but generally starts by putting the intestines through a wringer to squeeze out the mucosal tissue as a kind of pulp.
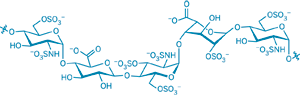
The antithrombin-III binding domain of heparin illustrates the chemical variety of the drug.
Heparin producers mix the pulp with an anion exchange resin to capture negatively charged material. They add salt to release the heparin from the resin, and then alcohol to precipitate out crude heparin, which is filtered and dried. The producers then subject crude heparin to further rounds of purification through treatment with proteases, nucleases, and heating to inactivate pathogenic material. The resulting material may go through additional ion-exchange or precipitation steps, as well as treatment to remove low-molecular-weight materials.
NOTABLY, the long chains of heparin with all their chemical diversity remain largely intact. Standard heparin, also called unfractionated heparin, is not purified to the point that only the pentasaccharide specific for AT-III binding remains. Even for LMWH, the chains are merely shortened. "You take this great mixture and introduce it into living systems, and you have a multitude of responses," Seeberger comments. "Obviously there's some anticoagulant activity, but there are all sorts of other things you're going to hit as well."
At the end of December 2007, Baxter noticed an increase in reports of allergic reactions in patients treated in dialysis centers using multidose heparin products. In January, Baxter recalled specific lots associated with those reactions, and on Feb. 28, it expanded the recall to include all of its heparin products. On March 5, FDA reported that it had received a total of 785 reports of adverse events related to heparin since Jan. 1, 2007, including 19 deaths that fit a specific profile of anaphylaxis and hypotension.
A "heparin-like" contaminant was found in lots of Baxter's heparin associated with adverse reactions; on March 19, FDA announced the contaminant had been identified as hypersulfated chondroitin sulfate (see page 13). Baxter receives heparin active pharmaceutical ingredient (API) from Scientific Protein Laboratories (SPL); all of the contaminated heparin came from SPL's Changzhou facility in China. The implicated API passed all standard U.S. Pharmacopeia regulatory tests, including potency assays, yet contained as much as 50% contaminant. On March 14, FDA announced that all heparin imported into the U.S. must be tested by nuclear magnetic resonance spectroscopy and capillary electrophoresis, either by the importing companies or by FDA.
No one has determined yet whether the contaminant was added to products accidentally or intentionally. Much attention is focused on China, where the crude heparin originated and regulatory oversight may be lacking. Three Japanese companies have recalled heparin sourced from SPL. The German company Rotexmedica GmbH, a unit of the French company Groupe Panpharma, has recalled heparin that originated in China, although not from SPL, and that has been associated with adverse reactions in Germany.
The identification of chondroitin sulfate in heparin comes a year after melamine was discovered in pet food. An industrial compound used in thermosetting plastics, melamine would have given the illusion of higher protein content in the food. The heparin revelations perhaps illustrate a need not only for more rigorous inspections of imported food and health care products, but also more advanced testing to ensure products are untainted by chemically similar mimics.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter