Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Little-Ring Metathesis
January 28, 2008
| A version of this story appeared in
Volume 86, Issue 4
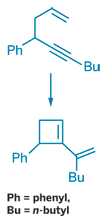
Ring-closing metathesis has been used to establish a new route to functionalized cyclobutenes, a surprising result given the generally accepted idea that ring strain precludes formation of small cyclic rings using this approach (J. Am. Chem. Soc. 2008, 130, 1562). Ring-closing metathesis is popular for synthesizing medium and large rings from diene or enyne precursors. But strained cyclopropenes and cyclobutenes can easily be reopened by the carbene catalyst. In fact, cyclobutenes are starting materials for ring-opening metathesis polymerization and other metathesis reactions. However, Olivier Debleds and Jean-Marc Campagne of Institut Charles Gerhardt Montpellier, in France, surmised that the orientation of 1,5-enynes previously made in their lab might lead to less reactive cyclobutenes under the appropriate conditions. By using the Hoveyda-Grubbs second-generation ruthenium catalyst, the researchers were able to prepare several cyclobutenes (one example shown). They subsequently used the cyclobutene shown in a Diels-Alder reaction with a cyclic azo derivative to form a tricyclic compound, a reaction that could prove useful in natural products synthesis.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter