Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Peeking At Reactions
NMR method visualizes hydrogenation reactions inside microreactor
by Celia Henry Arnaud
January 28, 2008
| A version of this story appeared in
Volume 86, Issue 4
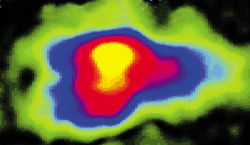
The NMR signal of propane formed by hydrogenation of propene with p-H2 is 300-fold stronger than the NMR signal of propene.
MANY INDUSTRIAL PROCESSES rely on catalytic hydrogenation. Pinpointing the active parts of a catalyst bed could help optimize those processes. A new nuclear magnetic resonance (NMR) imaging method allows researchers to visualize where hydrogenation reactions occur as gas-phase reactants flow through a microreactor (Science 2008, 319, 442). The method could be used as a tool for catalyst development and microreactor characterization.
The method was developed by a team led by chemistry professor Alexander Pines and postdoctoral associate Louis-S. Bouchard of Lawrence Berkeley National Laboratory and the University of California, Berkeley. The researchers use the para form of hydrogen to amplify the NMR signal of the product of a catalytic hydrogenation reaction.
p-H2, a spin isomer of molecular hydrogen in which the magnetic spins of the two protons are aligned in opposite directions, has no observable NMR signal on its own. But when the two hydrogen atoms participate in a pairwise hydrogenation reaction, they become magnetically inequivalent. The result is a polarized product with an enhanced NMR signal.
The researchers use the method to observe the hydrogenation of propene to propane inside a microreactor. The signal from the polarized propane is 300-fold stronger than the propene signal. By mapping product formation, they can identify the active regions of the catalyst bed.
Although the method requires p-H2 to be one of the reactants, it "allows you to study an even broader class of chemical reactions," Bouchard says. With the judicious selection of radio-frequency pulses, the researchers can extend the lifetime of the polarized product so that it can be used in other reactions. For example, a polarized alkene formed by the hydrogenation of an alkyne could be used to study polymerization reactions.
M. Daniel Raftery, a chemistry professor at Purdue University who develops NMR methods, calls the research "very impressive work." The method "should provide tremendous information for modeling studies," he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter