Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Expanding C-P Bond Chemistry
February 11, 2008
| A version of this story appeared in
Volume 86, Issue 6
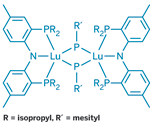
The first lanthanide-metal complex featuring a phosphinidene functional group has been reported by a team led by Jaqueline L. Kiplinger of Los Alamos National Laboratory (J. Am. Chem. Soc., DOI: 10.1021/ja7105306). This new compound class for the 4f elements could become "a platform for catalytic organophosphorus group-transfer processes and other C–P bond-forming reactions," Kiplinger says. The researchers made the complex by reacting a tridentate P–N–P pincer-type ligand with a lutetium precursor complex to form an isolatable intermediate, followed by treating the intermediate with mesitylphosphine (R′PH2). Unlike transition metals, which can form terminal phosphinidenes (M=P), the lutetium version only forms a dimer with bridging phosphinidene groups (shown). In preliminary reactivity studies, the dimer breaks up and reacts like a terminal phosphinidene, transferring the organophosphorus group to aldehydes and ketones to make phosphaalkenes, such as R′P=C(C6H5)2. "This phospha-Wittig chemistry suggests that we ultimately may be able to prepare a stable terminal lanthanide phosphinidene," Kiplinger adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter