Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Reaction Control
Selective bond breaking achieved with methane on a surface
by Elizabeth K. Wilson
February 11, 2008
| A version of this story appeared in
Volume 86, Issue 6
THE MUCH-SOUGHT-AFTER ability to pick and choose the bonds broken in a chemical reaction in the gas phase has been getting closer to becoming part of the chemists' repertoire over the past 20 years. In a forward jolt, researchers now have selectively broken C-H bonds in the catalytic dissociation of methane on a nickel surface.
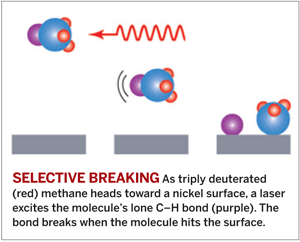
The control of such a complex reaction involving so many atoms had been thought to be intractable. But a team led by chemistry professor Arthur L. Utz of Tufts University used a laser to vibrationally excite C–H bonds in a beam of triply deuterated methane molecules, causing the C–H bonds to break as the molecules glance off the metal surface (Science 2008, 319, 790).
The research not only demonstrates the ability to control a reaction on a surface, but also reveals how energy flows during heterogeneous catalysis. This insight is important, because catalytic reactions of methane with metals are popular commercial and laboratory methods for producing H2.
Chemists have been seeking to selectively control bond breaking for decades. The first successful experiments involved simple reactions of deuterated water (HOD) with hydrogen atoms in the gas phase. Depending on the choice of energy, a bombarding laser could excite stretching in either the O–D or O–H bonds. An O–H stretch produced OD and H2, while an O–D stretch produced OH and HD.
A major impediment to progress in this area has been the tendency of the excitation energy to redistribute among a molecule's other bonds. Surface reactions have been thought to be even more problematic, as scores of surface atoms soak up the energy and thwart the bond-breaking process.
The Utz group overcame the problem by shooting triply deuterated methane molecules from a fast-moving beam at a nickel surface while exciting C–H bond stretches with a laser. The combination of translational and vibrational energy allowed the surface-catalyzed reaction to happen quickly, before the energy had a chance to redistribute.
The work is "a lovely additional example of bond-selective chemistry," says Richard N. Zare, chemistry professor at Stanford University.
The work's "punch line is that one can perform bond-selective chemistry even in the face of extensive interactions with the surface," says F. Fleming Crim, chemistry professor at the University of Wisconsin, Madison, and a bond-selective chemistry pioneer.
Even a few years ago, Utz says, chemists believed the great number of surface atoms in such a complex reaction would prevent control of bond breaking. Utz, who was a graduate student of Crim's during the early years of selective chemistry, notes, "We surprised ourselves with the extent to which we could control reactivity on a metal surface."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter