Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Guarding Against Too Much Light
Protective mechanism, which has inspired synthetic system, depends on minor constituents of photosynthetic apparatus
by Celia Henry Arnaud
May 8, 2008

By studying and mimicking photosynthesis, researchers hope to unlock and harness the secrets of how plants protect themselves from too much sunlight. The work might have application in the development of energy-efficient solar cells.

At Arizona State University, a team of researchers has designed a self-regulating molecule that boosts or slows down a charge transfer process in response to changes in light intensity. Meanwhile, a team at Lawrence Berkeley National Laboratory (LBNL) and the University of California, Berkeley, is closer to figuring out the details of the natural protective mechanism.
In photosynthesis, an ultraefficient charge transfer reaction converts virtually all incoming light energy into electrochemical energy. But such efficiency can be dangerous to the plant when there's too much light. "If the light gets too bright, then the reaction center works too fast and produces a lot of high-energy intermediates that don't have anywhere to go because the system is jammed up down the line," says Devens Gust, one of the leaders of the Arizona State team.
Plants keep themselves from burning out through nonphotochemical quenching, that is, by dissipating excess absorbed energy as heat. Inspired by this process, Gust and his coworkers designed a molecule that converts absorbed light to electrochemical energy but reduces the efficiency of the conversion, its quantum yield, as the light intensity increases.
The molecule consists of two light-gathering antennas, a porphyrin electron donor, a fullerene electron acceptor, and a control unit that reversibly photoisomerizes between a dihydroindolizine (DHI) and a betaine (BT) (Nat. Nanotechnol., DOI: 10.1038/nnano.2008.97). The DHI form doesn't effect the electron transfer between the porphyrin and the fullerene. In contrast, the BT form is "able to suck the excitation energy out of the porphyrin antenna system," thereby inhibiting electron transfer, Gust says.
At low light levels, most of the molecules are in the DHI form, and the system has a quantum yield of 82%. At higher light intensities, more of the molecules convert to the BT form, reducing the electron-transfer quantum yield to as low as 27%. When the light intensity goes back down, the molecule reverts to the DHI form.
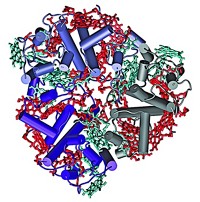
In the natural system, nonphotochemical quenching depends on a number of components. Scientists don't understand how these components interact, although they know that a pH gradient triggers the process. The mechanism previously proposed is that energy is transferred from chlorophylls bound to LHCII, the major chlorophyll-containing antenna complex, to a chlorophyll-zeaxanthin heterodimer. The question has been: Where is the heterodimer located?
Graham R. Fleming and coworkers at LBNL find that the quenching occurs in the minor antenna protein complexes CP24, CP26, and CP29 to dissipate excess absorbed light energy in higher plants (Science 2008, 320, 794). The researchers also find that the chlorophyll molecules in CP29 that participate in the charge-transfer process are a tightly coupled pair at the antenna protein's A5 and B5 binding sites.
"I was surprised that it was so clear that quenching was only happening in these minor complexes and not in LHCII," Fleming says. The researchers propose that pH-dependent conformational changes alter the strength of coupling between the two chlorophyll molecules and induce quenching.
Understanding how this protective mechanism works could lead to better synthetic systems for solar energy conversion. "Just one simple feedback system is certainly not how the natural system works," Fleming says. "Working through all the components and modeling them properly will lead us to have much more sophisticated ideas about how to make light-driven systems that work optimally."





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter