Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Protein Folding
Molecular crowding causes disease protein to change shape
by Stu Borman
August 13, 2008

New findings on the effects of solution “crowding” on protein shape could help researchers better understand how proteins function in the jam-packed cellular environment.
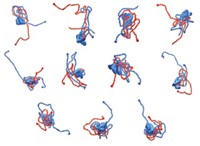
Computer simulations and in vitro experiments on the football-shaped protein VlsE from the Lyme disease bacterium reveal that increased crowding by surrounding macromolecules causes the protein to change shape. The protein’s shape-shift is so dramatic that a hidden site recognized by antibodies used in Lyme disease diagnostics pops out like a jack-in-the-box. The work was done by Margaret S. Cheung at the University of Houston, Pernilla Wittung-Stafshede at Rice University, and coworkers (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.0803672105).
“We propose that shape changes may be one way nature controls protein function, much like different facets can be exposed in an origami ‘cootie catcher,’ ” says Wittung-Stafshede, now at Umeå University, in Sweden. “It may be possible to develop targeted crowding as a precise tool to manipulate protein conformations to turn on or off specific activities and signaling cascades.”
In 1981, physical biochemist Allen P. Minton of the National Institute of Diabetes & Digestive & Kidney Diseases used a model system to first predict that crowding could cause elongated proteins to adopt more compact shapes. And other early studies showed that crowding could influence protein stability and folding speed. But the new study is the first to demonstrate crowding-induced shape changes in a large, biologically relevant protein. “I am pleased to see an experimental demonstration of the effect,” Minton says.
“What is new here is the idea that if a native protein has an elongated or nonspherical shape, then molecular crowding agents can selectively stabilize various states,” comments biophysics professor Ken A. Dill of the University of California, San Francisco. The study is “a significant extension of Minton’s early predictions,” and it shows that crowding “may have interesting biological relevance.”
Cheung, Wittung-Stafshede, and coworkers show that as crowding increases, VlsE converts from its native football shape to a bent-bean structure with additional α-helices. Then, at a level of crowding comparable with that in living cells, some of the α-helices change to β-sheet-like conformations, and the protein collapses to become spherical.
In the spherical state, an antibody-recognition site that had been largely hidden in the football and bent-bean forms becomes exposed on the outside of the protein. Until now, researchers didn’t understand how antibodies used in diagnostic tests for Lyme disease interacted with and recognized this site, which appeared inaccessible in crystal structures of native VlsE.
“Crowding and other environmental factors that affect protein shape may modulate protein function in cells,” says biological physicist José Onuchic of UC San Diego. “Much more is needed to demonstrate the feasibility of this effect,” but the study suggests a new mechanism cells may use to modulate protein function, he adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter