Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
An Easier Way To Model Hydrate Lattices
New method streamlines computations by focusing on likely structures
by Elizabeth K. Wilson
June 1, 2009
| A version of this story appeared in
Volume 87, Issue 22

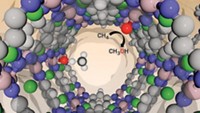
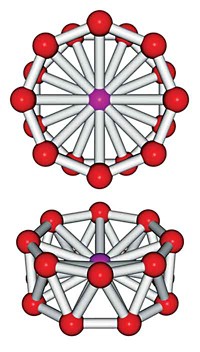
Hydrate lattices, which are made up of networks of water molecule cages, hold promise as a storage medium for hydrogen and other chemical species. At the moment, computer modeling of these structures to search for the best storage hydrates is generally cumbersome because of the millions of potential configurations the network of hydrogen bonds can assume. Now, Pacific Northwest National Laboratory chemist Sotiris S. Xantheas and colleagues have devised an approach that greatly streamlines calculation of the most likely unit cell structures of hydrate lattices (J. Am. Chem. Soc., DOI: 10.1021/ja9011222). The PNNL group focused on a hydrate lattice family composed of unit cells made from six cages that contain 24 water molecules and two cages that contain 20 water molecules. They first eliminated higher energy configurations from the list of possible cage networks by maximizing the number of strong hydrogen bonds. With a list of 3 million structures pared down to 321, the group then computationally built up the networks of the three-dimensional lattices. The method should help researchers design other hydrate lattices and focus their energies on hydrates they want to try to make, the authors write.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter