Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New Bond In Biology
Biochemistry: Sulfilimine connection toughens tissue
by Sarah Everts
September 7, 2009
| A version of this story appeared in
Volume 87, Issue 36
The mechanical strength of epithelial tissue found in multicellular organisms as diverse as fruit flies, worms, and humans is likely bolstered by a type of covalent bond that has never before been seen in biological tissue, according to a new report.
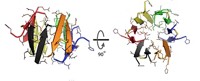
Researchers led by Billy G. Hudson, a biochemist at Vanderbilt University, show that collagen IV protein networks—which provide structural stability to tissue—are reinforced by the newly unearthed sulfilimine bond: S=.N (Science 2009, 325, 1230).
This “highly unusual cross-link” is a “very exciting discovery,” comments Hans Peter Bächinger, a biochemist who studies collagen proteins at Shriners Research Center, in Portland, Ore.
In epithelial tissue, which covers bodily cavities and glands, three strands of collagen IV assemble into ropelike helical twists, forming the equivalent of a brick in the proteinaceous scaffolding known as the extracellular matrix that surrounds cells. Researchers have known for decades that these collagen bricks are connected chemically by some sort of “mortar,” but the nature of that mortar remained a puzzle, Hudson says.
Using nuclear magnetic resonance and mass spectrometry, Hudson’s team discovered that methionine and hydroxylysine residues at the ends of each of the three collagen strands in the helical twist are chemically cross-linked by a sulfilimine bond to other strands in a nearby collagen trimer. The three sulfilimine connections bundle together six collagen IV molecules.
Bächinger notes that “the discovery poses additional questions,” such as whether the bond is formed by an enzymatic reaction and which enzymes do the work. Pinpointing the enzymes, Hudson says, may provide clues to the origins of a rare autoimmune disease called Goodpasture’s syndrome, which is characterized by bleeding in a patient’s lungs.
Besides acting as structural reinforcement, collagen IV’s sulfilimine bonds seem to block the immune system from reaching inside the extracellular matrix, the consequence of which kindles Goodpasture’s syndrome, Hudson adds. He suspects loss or malfunction of the enzymes that form the bond may be the molecular cause of this disease.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter