Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Pharma Outsourcing
Relationships between drug companies and chemistry outsourcing partners can span years, even decades
by Michael McCoy
March 15, 2010
| A version of this story appeared in
Volume 88, Issue 11

Economists are calling the past 24 months or so the Great Recession, but you wouldn’t know it during a visit to a typical drug discovery lab or pharmaceutical chemical manufacturing plant.
COVER STORY
Pharma Outsourcing
That’s because the discovery, development, and commercialization of new active pharmaceutical ingredients is a multiyear endeavor that transcends business cycles. From initial discovery to market launch, the process of creating a new drug can encompass multiple economic ups and downs.
Of course, the drug industry is not immune to the vicissitudes of economic fortune. Small biotech firms depend on venture capitalists to provide funding, and funds dry up during periods of financial hardship. Even profitable big drug companies seem more inclined to cut back when the business world around them is doing the same.
But for many pharmaceutical molecules with good efficacy and promising market potential, the recent recession was little more than background noise. The backers of these molecules had the foresight—and luckily the money—to stick with them.
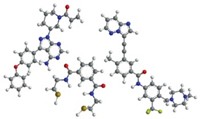
In the pages that follow, C&EN presents case studies of three pharmaceutical molecules and the outsourcing partnerships that have shepherded them during the latest downturn, and previous ones, as well.
In the first case, a biotechnology firm enlists the aid of a pharmaceutical chemical manufacturer to find a synthetic route to a molecule better than its own cumbersome medicinal chemistry process. That relationship got its start in 2000 and, despite one big setback, continues today.
The second case chronicles a molecule that was developed by a hospital, licensed to a multinational drugmaker, and then turned over to a biotech firm, which finally won Food & Drug Administration approval in January. One chemical maker stood by the molecule for more than 25 years, which is a long time even by drug industry standards.
In the third case, a contract manufacturer decides to step outside its usual role as hired hand and develop a molecule on its own. The outsourcing company became the outsourcer, partnering with a technology firm for process chemistry and a big drugmaker for legal and marketplace assistance. Because the molecule is a generic drug, the timeline is relatively short: five years and counting.
For the economy at large, even five years is a lifetime. For companies involved in drug discovery and development, however, five years is just getting started.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter