Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Protected Oxidation Catalyst
A homogeneous cobalt polyoxometalate catalyst splits water while avoiding oxygen-promoted degradation
by Elizabeth K. Wilson
March 15, 2010
| A version of this story appeared in
Volume 88, Issue 11
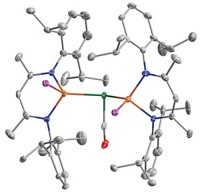
A new catalyst that breaks water into its oxygen and hydrogen constituents avoids a problem that has plagued previous cobalt-based water-oxidation catalysts—the propensity of the released O2 to destroy the catalyst. Chemists have been avidly searching for photosynthetic mimics that catalyze the oxidation of water, with the goal of creating H2 to power fuel cells. The new catalyst, a bulky complex with the formula [Co4(H2O)2(PW9O34)2]10–, comes from the lab of Craig L. Hill at Emory University (Science, DOI:10.1126/science.1185372 ). Hill and coworkers say this complex is now the fastest known homogeneous water-oxidation catalyst and is an improvement over popular heterogeneous cobalt-based water-oxidation catalysts. Hill’s group surrounded the catalyst’s cobalt oxide core with bulky polytungstate ligands that resist oxidation. Not only is the catalyst free of carbon-based ligands, which are also vulnerable to oxidation, but it also self-assembles in boiling water. That the elements in the catalyst—cobalt, tungsten, phosphorus, and oxygen—are cheap and abundant is another plus. The researchers say their work prompts the examination of other polyoxometalate-stabilized multi-transition-metal oxide clusters as water-oxidation catalysts.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter