Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Thorium Complex Almost Maxes Out
Inorganic Chemistry: 15-Coordinate thorium aminodiboranate sets a record, just one bond short of a perfect 16
by Stephen K. Ritter
April 5, 2010
| A version of this story appeared in
Volume 88, Issue 14
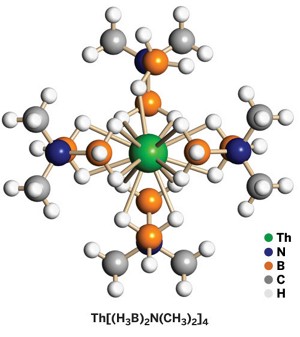
A multidisciplinary research team has reported the first example of a stable, crystallographically characterized 15-coordinate metal complex, an achievement that extends the boundaries of chemical bonding.
The thorium aminodiboranate created by Gregory S. Girolami of the University of Illinois, Urbana-Champaign, and colleagues bests the prior record of 14 two-electron metal-ligand bonds in complexes such as U(BH4)4. But for one errant hydrogen atom, the molecule's metal could have had 16 bonds, the theoretical maximum based on the number of atomic orbitals thorium has available for bonding; some metallocenes can have more metal-ligand contacts but at most 12 two-electron metal-ligand bonds.
The trick to reaching high coordination numbers is combining a large metal cation with small chelating ligands. Girolami and graduate student Scott R. Daly accomplished that by pairing thorium, an f-block element with a large covalent radius, and hydrogen, the smallest of ligands. They made the 15-coordinate Th[(H3B)2N(CH3)2]4 by treating ThCl4 with Na[(H3B)2N(CH3)2] in tetrahydrofuran, isolating it as colorless crystals (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200905797).
On the basis of X-ray and neutron diffraction and other characterization techniques, the team determined that two hydrogen atoms from each boron in three of the ligands coordinate to thorium via H–B–H chelating bridges. In the fourth ligand, one hydrogen atom fails to link with thorium. The researchers believe steric crowding distorts the molecule's framework so that the hydrogen is just out of reach.
Quantum chemical calculations on the thorium complex performed by Laura Gagliardi's group at the University of Minnesota, Minneapolis, indicate that in the gas phase all 16 hydrogen atoms should be coordinated. Those results hint that solid-state 16-coordinate complexes are possible if the right metal-ligand combination can be determined.
The new thorium complex "is truly fascinating and the results are very convincingly presented," says Roland A. Fischer of Ruhr University, in Bochum, Germany, an expert in high-coordination chemistry. "The key to this record is choosing the smallest chelating ligand and taking advantage of the flexible binding of the BH3 units. It remains a challenge to achieve a coordination number greater than 12 for ligands without such bridging groups."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter