Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chiral Sulfide Boosts Ylide Chemistry
Inexpensive isothiocineole demonstrates high selectivity in asymmetric syntheses of epoxides and aziridines
by Bethany Halford
February 8, 2010
| A version of this story appeared in
Volume 88, Issue 6
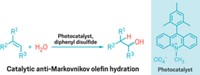
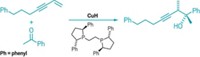
Sulfur ylides can be used in the asymmetric syntheses of epoxides and aziridines, but they rarely are because the reactions haven’t been capable of producing synthetically useful compounds with high diastereo- and enantioselectivity. Furthermore, the strategy usually requires a tedious multistep synthesis to make a chiral sulfide as the ylide precursor. Now, Varinder K. Aggarwal and coworkers of the University of Bristol, in England, have developed a chiral sulfide that gets around both problems (J. Am. Chem. Soc., DOI: 10.1021/ja9100276). By heating elemental sulfur with limonene in the presence of γ-terpinene, they produce isothiocineole, a chiral sulfide that costs less than $1.00 per gram to make. Aggarwal’s team found isothiocineole exhibits outstanding selectivities in sulfur ylide-mediated asymmetric epoxidations and aziridinations. The researchers simply convert the sulfide to a benzylic or allylic sulfonium salt and react it with aldehydes or imines to produce epoxides or aziridines, respectively, with “perfect enantioselectivities and the highest diastereoselectivities reported to date,” the researchers say. The isothiocineole, they write, “should change sulfur ylide-mediated epoxidation into a scalable, mainstream practical method.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter