Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Novel Copper-Nitrosyl Complex Synthesized
Inorganic Chemistry: Species is similar to key intermediate in enzymatic nitrite reduction
by Jyllian N. Kemsley
October 5, 2010
In work that could help researchers better understand how nitrogen is converted into different forms on a worldwide scale, a group at the University of California, Santa Barbara, has synthesized and characterized a novel copper-nitrosyl complex (J. Am. Chem. Soc., DOI: 10.1021/ja105930b).
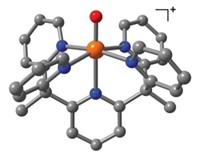
The complex incorporates a Cu(II)–NO• center, the radical species that is believed to be a key intermediate in the reduction of NO2– to NO by the enzyme copper nitrite reductase. The enzyme is found in a variety of bacteria and plays a key role in the global nitrogen cycle.
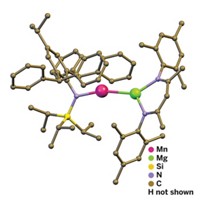
Chemistry professor Trevor W. Hayton, graduate student Ashley M. Wright, and staff crystallographer Guang Wu prepared the new complex by adding NOPF6 to copper metal powder in nitromethane. Analysis of crystals formed from the resulting deep purple solution showed that the product was a distorted octahedral copper complex with five CH3NO2 ligands and one NO ligand. The nitrosyl group is bound in a bent geometry. The Cu–N bond length of 1.955 Å is significantly longer than those of other Cu–NO complexes, indicating a weak Cu–NO interaction in the new complex, the group reports.
The nitromethane ligands don't precisely mimic the histidine and water ligands bound to copper in the enzyme. Nevertheless, the synthesis and characterization of the complex could provide insight into the detailed mechanism of action of copper nitrite reductase, as well as the mechanisms of other NOx reductive processes promoted by copper centers, says William B. Tolman, a chemistry professor at the University of Minnesota.
In addition to their synthetic and structural work on the complex, Hayton and colleagues found that it can transfer the nitrosyl group, as NO+, to a variety of substituted benzene compounds. The group is continuing to characterize the electronic structure and reactivity of the complex, Hayton says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter