Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Sticking Fast To Foil Hepatitis C
Biochemistry: Aiming beyond the active site of a virus's key protease yields selective blockers
by Carmen Drahl
December 1, 2010

By targeting a noncatalytic cysteine, researchers have designed selective irreversible blockers of a protease enzyme essential for hepatitis C virus replication (Nat. Chem. Biol., DOI: 10.1038/nchembio.492).
The work is the first demonstration that steering clear of the active site is a viable design strategy for drugs that react to form a covalent bond to proteases, a broad class of proteins that includes many drug targets. This strategy has already proven useful for blocking other proteins such as kinases.
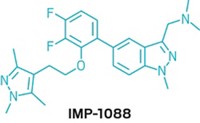
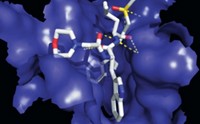
A team from the biotechnology company Avila Therapeutics developed the new protease blockers, which covalently bind to a cysteine in hepatitis C protease's substrate-binding site. In contrast, most other hepatitis C protease inhibitors in development reversibly bind to a catalytic amino acid common to proteases in human hosts as well as in viruses. Avila's published inhibitor structures are prototypes, but the company hopes to begin human clinical trials with optimized compounds in 2011. The team says its comprehensive structural analysis of hundreds of proteases suggests this strategy can be applied broadly.
However, it's not clear how applicable the team's strategy will be to all proteases, says Matthew S. Bogyo of Stanford University, who develops chemical tools to study the roles of proteases in disease. Nevertheless, "covalent inhibitors benefit from a long duration of action, and when used for clearing pathogens such as hepatitis C virus, they may make more sense than classical reversible inhibitors," Bogyo says. Covalent inhibitors can also make several aspects of drug development more straightforward because it's easier to monitor their target selectivity and distribution throughout the body compared with reversible inhibitors, he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter