Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Improved Route To OCF3 Building Blocks
Versatile strategy improves the synthesis of aliphatic organic molecules containing trifluoromethoxy groups
by Stephen K. Ritter
January 3, 2011
| A version of this story appeared in
Volume 89, Issue 1

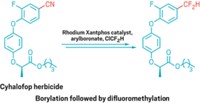
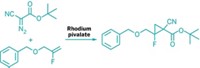
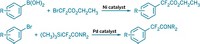
A new synthetic strategy for introducing the trifluoromethoxy group into aliphatic organic molecules is a promising way to make pharmaceutical and agricultural chemicals, according to Karsten Koppe of Heinrich Heine University, in Düsseldorf, Germany. The OCF3 group is strongly electron withdrawing and offers advantages such as increased lipophilicity over popular F or CF3 substituents that are commonly used to improve drug efficacy. Although an industrial process using chlorine-fluorine exchange to prepare OCF3-containing aromatic compounds exists, there are few examples of procedures to make aliphatic compounds bearing OCF3 groups, and they are limited in scope, Koppe said. Koppe and coworkers, in collaboration with Nikolai V. Ignat’ev of Merck KGaA, in Darmstadt, Germany, devised a method that uses OCF3 salts to introduce OCF3 to aliphatic compounds bearing different functional groups. For example, the researchers prepared KOCF3 by adding CF3SO2OCF3 to KF in N,N-dimethylacetamide solvent. Adding organic halides or mesylates to KOCF3 results in the trifluoromethoxy compounds, which can be derivatized to create epoxides, diols, aminoalcohols, and other intermediates, Koppe said, “giving us a whole factory of OCF3 building blocks.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter