Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
'Good' Cholesterol Comes Into Focus
Heart Disease: Cross-linking mass spectrometry gives clues to native structure of high-density lipoprotein
by Celia Henry Arnaud
March 21, 2011
| A version of this story appeared in
Volume 89, Issue 12

High-density lipoprotein—the so-called good cholesterol—plays an important role in reducing the risk of heart disease. Despite its importance, all structural information has come from synthetic HDL particles. Now, biochemists at the University of Cincinnati have obtained the first structural information on HDL isolated from human plasma (Nat. Struct. Molec. Biol., DOI: 10.1038/nsmb.2028).
An understanding of HDL's structural organization "will be highly significant for future development of drugs affecting the anti-inflammatory, antioxidant, and antiatherogenic functions of HDL that could lead to new approaches to preventing and reversing coronary heart disease," says Jere P. Segrest, a researcher studying lipoprotein structure at the University of Alabama, Birmingham.
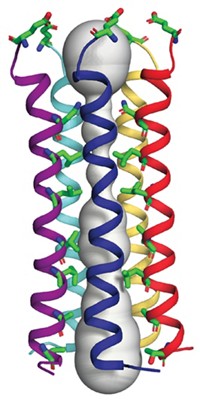
HDL particles aren't suited to high-resolution methods like X-ray crystallography or NMR. W. Sean Davidson and coworkers instead turned to mass spectrometric analysis of cross-links between lysines to obtain distance constraints they could use to model the most abundant protein in HDL particles, apolipoprotein A-1 (ApoA-1).
Synthetic HDL particles—which are built in the lab from HDL's protein parts—typically contain only three ApoA-1 chains, but the HDL particles that Davidson and coworkers isolated were larger, containing four or five chains. The cross-linking patterns, which are similar regardless of the number of protein chains, suggest that ApoA-1 adopts a symmetric cagelike structure. Size differences between particles with the same number of chains appear to be due to the twisting of protein chains while maintaining the same contacts.
"Surprisingly and importantly, the chemical cross-linking and mass spectrometry results show that ApoA-1 maintains distinct intermolecular contacts regardless of HDL particle size and shape," says Michael C. Phillips, an HDL researcher at Children's Hospital of Philadelphia.
Even such low-resolution structures could help scientists figure out good places to mutate on the protein to figure out how it functions, Davidson says. "That's going to take us a long way toward understanding how HDL is protective against cardiovascular disease and will help take us to the next step of designing ways to mimic it."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter