Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Heterogeneous Tandem Catalysis
Catalysis: Nanostructured layered material performs multistep reaction
by Elizabeth K. Wilson
April 18, 2011
| A version of this story appeared in
Volume 89, Issue 16
A novel type of layered nanocrystal catalyst that promises a new way to perform multistep, multicatalyst reactions could make industrial processes more efficient and environmentally friendly.
University of California, Berkeley, chemistry professors Peidong Yang and Gabor A. Somorjai and their colleagues report the design of a class of nanocrystal catalysts that consist of two catalysts stacked on top of each other (Nat. Chem., DOI: 10.1038/nchem.1018). They show that the layered material can catalyze the industrially important multistep process of producing propanal from methanol and ethylene.
The work has drawn praise from experts in the field, including catalysis expert R. Tom Baker of the University of Ottawa. “This clever design of nanostructured catalysts with interface control successfully realizes an important tandem reaction sequence,” Baker says.
Catalyst makers often place heterogeneous metal catalysts on materials such as metal oxides to help increase their surface area. But as the researchers note, the interfaces between a metal and a metal oxide can also be key catalytic moieties in and of themselves.
Consequently, scientists seek to harness the catalytic power of these interfaces. That, coupled with progress in nanostructure-assembling techniques, prompted the Berkeley group’s development of the new multilayered catalyst.
Although a few tandem catalysts have been synthesized before, these have been for homogeneous catalytic systems. Compared with homogeneous systems, however, heterogeneous catalysts are more stable and are easier to separate from products.
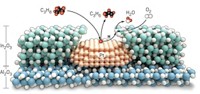
To build their heterogeneous tandem catalyst, the Berkeley group deposited single layers of platinum nanocubes on top of a silica base. They then added a single layer of cerium oxide nanocubes to form an array of bilayered cubes 6–8 nm on a side. The interface between CeO2 and Pt catalyzed the decomposition of methanol to CO and H2. The Pt-SiO2 interface then catalyzed the reaction of CO and H2 with ethylene to form propanal. This tandem reaction produced propanal even faster than the traditional method of starting with CO and H2 and using a Pt-SiO2 catalyst.
“This is clearly an example where the system is greater than the sum of the parts,” says catalysis expert Christopher B. Murray of the University of Pennsylvania. He notes that the method has two strengths: Scientists can control the crystallographic orientation of the components by using cubic building blocks, and they can also control the spatial relationship of the components with layer-by-layer deposition. Both features allow fine-tuning of the catalyst’s reactivity.
Even more promising, adds catalysis expert François-Xavier Felpin of the University of Bordeaux, in France, is the possibility, raised by this work, of “creating materials with unexpected and novel electronic properties.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter